Rice blast is one of the most destructive diseases affecting rice production worldwide. The development and rational use of resistant varieties has been the most effective and economical measure to control blast. In this review, we summarized the cloning and utilization of rice blast resistance genes, such as Pi1, Pi2, Pi9, Pi54, Pigm and Piz-t. We concluded that three main problems in the current breeding of rice blast resistance are: availability of few R (resistance) genes that confer resistance to both seedling and panicle blast, the resistance effect of pyramided lines is not the result of a simple accumulation of resistance spectrum, and only a few R genes have been successfully used for molecular breeding. Therefore, novel utilization strategies for rice blast R genes in molecular breeding were proposed, such as accurately understanding the utilization of R genes in main modern rice varieties, creating a core resistant germplasm with excellent comprehensive traits, screening and utilizing broad- spectrum and durable resistance gene combinations. Lastly, the trends and possible development direction of blast resistance improvement were also discussed, including new genes regulating resistance identified via GWAS (genome-wide association study) and improving rice blast resistance using genetic editing.
Rice blast, caused by Magnaporthe oryzaeB.C. Couch, is one of the most destructive diseases encountered in rice production. Once rice is attacked by M. oryzae, pattern recognition receptors (PRRs) on the cell surface can specifically recognize pathogen-associated molecule patterns (PAMPs), and activate defense response by cell wall modification, callose deposition, and via expression of defense-related proteins in host cells, which is termed as PAMP-triggered immunity (PTI) (Jones and Dangl, 2006). However, PTI is a weak and non-specific resistance mechanism (Bernoux et al, 2011; Segonzac and Zipfel, 2011). In many cases, M. oryzae can secrete certain effectors to inhibit PAMP- induced PTI and can break resistance responses (Jones and Dangl, 2006; Birch et al, 2009; Block and Alfano, 2011; Mentlak et al, 2012). At the same time, rice has acquired more specific resistance proteins that directly or indirectly recognize pathogen effector proteins. This recognition mechanism activates a second layer of the defense response in rice, known as effector- triggered immunity (ETI), which results in the production of ion (Ca2+, K+ and H+) currents, superoxide, nitric oxide, and programmed cell death at the site of invasion (Dangl et al, 1996; Nurnberger et al, 2004). ETI is a highly specialized disease resistance mechanism in the host (Boller and He, 2009), which is activated in the gene-for-gene model upon recognition by an R (resistance) protein of the corresponding effector protein of M. oryzae. Effector proteins are often encoded by avirulence genes in M. oryzae.
The R genes in rice correspond to the avirulence (AVR) genes in M. oryzae in a gene-for-gene manner (Flor, 1956), which ensures that the interaction between a specific R protein in rice and the corresponding AVR effector in the pathogen render resistant. To date, more than 40 AVR genes have been identified in M. oryzae, while 12 of them have been cloned. The cloned genes are PWL1(Kang et al, 1995), PWL2(Sweigard et al, 1995), AvrPi-ta (Jia et al, 2000), ACE1(Bö hnert et al, 2004), AvrPiz-t (Li et al, 2009), AvrPia (Miki et al, 2009), AvrPik/km/kp (Yoshida et al, 2009), Avr1-CO39(Cesari et al, 2013), AVRPii(Fujisaki et al, 2015), AVR-Pi9 (Wu J et al, 2015), AVRPib (Zhang et al, 2015) and AVRPi54 (Ray et al, 2016). The R protein encoded by R genes interacts directly or indirectly with the effector protein, thus sensing pathogen invasion and inducing disease resistance. Among the cloned AVR and R gene pairs, Pita/AvrPi-ta(Jia et al, 2000; Orbach et al, 2000), Pik/AvrPik(Yoshida et al, 2009; Kanzaki et al, 2012), Pia/AvrPia(Miki et al, 2009; Ortiz et al, 2017), Pi-CO39/Avr1-CO39(Cesari et al, 2013) and Pi54/AvrPi54(Ray et al, 2016) can interact directly with each other. While Pii/AVR-Pii(Fujisaki et al, 2015; Singh et al, 2016) and Piz-t/ AvrPiz-t(Park et al, 2012, 2016; Wang et al, 2016; Tang et al, 2017) require other proteins to complete the interaction. Despite the deployment of resistant varieties, blast epidemics can still occur, due to a lapse in host resistance and the emergence of new virulent pathotypes (Chuma et al, 2011). Several genetic events, including point mutations, insertion of transposable elements, deletion of partial or entire genes, etc, lead the function loss of AVR genes in M. oryzae (Li W T et al, 2019). Thus, the effectiveness of R genes highly dependents on the respective AVR gene. Deployment of blast resistant varieties requires regular monitoring of race dynamics, and current and future frequency of AVR genes across different regions (Selisana et al, 2017).
Currently, the uses of chemicals and resistant varieties are the main ways of rice blast management. In addition to increased costs, chemical control also causes serious environmental pollution and poses food safety risks. The use of R genes to breed resistant varieties remains the top-most economical and effective method to control rice blast (Wu Y Y et al, 2015). This review provided a summary of the identification, cloning and utilization of rice blast resistance genes, key problems in molecular breeding of rice to blast resistance and molecular breeding strategies based on cloned R genes. In addition, the trends and possible future development direction of blast resistance improvement were also discussed.
R genes are the foundation for disease resistance research and resistance breeding. The genetic analysis, gene mapping and cloning of rice blast resistance have been intensively studied. Since the first report of independently inherited three R genes Pia, Pii and Pik during 1960s (Yamasaki and Kiyosawa, 1966), more than 100 resistance genes or loci have been identified to date (Li W T et al, 2019; Li et al, 2020). R genes are distributed on 11 chromosomes of rice genome, except chromosome 3, and more than 64% are clustered in chromosomes 6, 11 and 12, representing 18%, 25% and 21%, respectively (Ashkani et al, 2016).
Since the cloning of first R gene, Pib, in 1999 (Wang et al, 1999), 31 R genes have been successfully cloned (Table 1). Except for pi21, which is a recessive R gene, the remaining 30 R genes are dominant. Among them, all genes except pi21, Pi35, Pi63, Pb1 and Pid3-I1 show complete resistance. Pi-d2 encodes a B-lectin kinase domain protein (Chen et al, 2006), while pi21 encodes a proline-rich protein with a heavy metal domain (Fukuoka et al, 2009), andPtr encodes an atypical protein with an armadillo repeat (Zhao et al, 2018). Remaining 28 R genes encode nucleotide- binding site leucine-rich repeat (NBS-LRR) domain proteins. Pik, Pikm, Pik-p, Pi1, Pike, Pi5, Pia and Pi-CO39 contain two NBS-LRR protein structural genes for blast resistance (Lee et al, 2009; Okuyama et al, 2011; Hua et al, 2012; Cesari et al, 2013). Pi5-1, Pb1, pi21 and Pi63 genes are induced by pathogen infection, while the remaining genes express constitutively. Majority of the cloned R genes induce resistance against leaf blast at the seedling stage, while only a few R genes, such asPb1, Pi25 and Pi64, confer resistance to panicle blast (Hayashi et al, 2010; Chen et al, 2011; Ma et al, 2015; Cao et al, 2019). Involvement of such a high numbers and types of R genes in rice blast resistance breeding applications indicates a complex genetics of this disease interaction.
| Table 1 List of cloned blast resistance genes. |
Higher variability inM. oryzae population and a frequent emergence of new virulent races cause a high selection pressure, resulting the resistant varieties often ‘ losing’ resistance within 3-5 years of cultivation and becoming susceptible. Therefore, integration of broad-spectrum and durable resistance has become a key issue among rice breeders (Wu et al, 2007). However, because of a higher number of R genes in rice, their deployment and utilization become an important challenge in blast resistance breeding to achieve broad- spectrum and durable resistance. Generally, two main strategies are deployed, the use of broad-spectrum resistance genes and gene pyramiding. However, some problems with these strategies remain to be solved.
Among the identified and cloned resistance genes, Pi1, Pi5, Pi33, Pi54, Piz, Piz-t, Pi2, Pi9, Pi40 and Pigm are broad-spectrum resistance genes to leaf blast. Pi1 in the LAC23 cultivar from West Africa shows resistance to 98% of 792 M. oryzae isolates in China (Chen et al, 2001). Pi5 shows resistance against 6 races from the Philippines, and 26 of 29 isolates from Korea (Jeon et al, 2003). Pi33, which interacts with AVR gene ACE1, is located on the short arm of chromosome 8, and shows resistance to over 2000 isolates originating from 55 countries (Berruyer et al, 2003). Pi54 identified in a highly resistant variety Tetep is also confirmed to have broad-spectrum resistance against predominant races found in India (Thakur et al, 2015). Six R genes, Pi2, Pi9, Pi40, Pigm, Piz-t and Piz, harbor alleles of the Piz locus located on the short arm near the centromere of rice chromosome 6 (Qu et al, 2006; Zhou et al, 2006; Jeung et al, 2007; Deng et al, 2017). The Pizgene cluster from the US cultivar Zenith shows resistant to five US races (IH-1, IG-1, IC-17, IE-1 and IE1k) (RoyChowdhury et al, 2012). Pi9 confers a high level of resistance to 43 isolates collected from 13 countries (Qu et al, 2006). Pi2 was cloned from a gene cluster composed of nine gene members (named Nbs1-Pi2 to Nbs9-Pi2) encoding proteins with an NBS-LRR structure (Zhou et al, 2006). Lines carrying Pi2 confer resistance to 455 isolates collected from different regions of the Philippines and most of the 792 isolates from the 13 major rice regions of China (Chen et al, 1996). Piz-t strongly resembles to Pi2 in sequence and structure having only eight amino-acid differences within the three leucine-rich repeats (Qu et al, 2006; Zhou et al, 2006). Pi40 from O. australiensis shows broad-spectrum resistance to rice blast races from South Korea (Suh et al, 2009). Pigm, a resistance gene from Gumei 4, a local variety in China, is resistant to 50 isolates from all over the world (Deng et al, 2017; Zeng et al, 2018). Bsr-d1, cloned from a Chinese local variety Digu, is an atypical resistance gene that encodes a C2H2 transcription factor protein, which exhibits similar phenotypic incomplete resistance to several races of rice blast (Li et al, 2017). However, it should be noted that the broad-spectrum resistance of the above-mentioned R genes was mostly evaluated in different genetic backgrounds, thus the resistant phenotypes might have been masked by other R genes.
Among various disease symptoms caused byM. oryzae, leaf blast and panicle blast are the most common. However, resistance to leaf and panicle blast is often inconsistent, and many varieties with high resistance to leaf blast at the seedling stage show susceptibility to panicle blast at the heading stage (Puri et al, 2009; Ishihara et al, 2014). Transcriptomic analysis showed that distinct defense-related gene expression is induced by leaf blast and panicle blast, suggesting that the genetic mechanisms of leaf blast and panicle blast resistance might differ and are independently controlled by different R genes (Liu et al, 2016). Disease evaluation via artificial inoculation at the seedling stage is a high throughput method with clear resistance/susceptibility phenotyping, which ultimately leads to most research focusing on leaf blast screening. However, less research has been carried out on the genetics of panicle blast resistance because of the increased fieldwork, complex phenotype evaluation system and high influence of environmental conditions for artificial inoculation testing. Presently, the evaluation of panicle blast resistance is mainly performed in disease nurseries under natural conditions. The above-mentioned broad-spectrum R genes such as Pi1, Pi2, Pi5, Pi9, Pi33, Pi40, Pi54, Pigm, Piz-t, Piz and bsr-d1 have not been evaluated using different isolates of M. oryzae by artificial inoculation at the heading stage. Therefore, it is not clear whether they exhibit broad-spectrum resistance to panicle blast. Recently, Wu et al (2016, 2017) constructed a set of near-isogenic lines (NILs) for six resistance alleles of the Piz locus (Pi2, Pi9, Pi40, Pigm, Piz-t and Piz) in the genetic background of the indica rice Yangdao 6 and japonica rice 07GY31. Using an improved method of artificial inoculation, the panicle blast resistance evaluation of these NILs was carried out with representative isolates of M. oryzae collected from different ecological regions of China. Only Pigm show stable broad-spectrum resistance to leaf blast and panicle blast in the genetic background of indicaandjaponica rice (Wu et al, 2016, 2017). However, Pi2, Pi9, Pi40, Piz-t andPiz only show specific resistance to the blast population in some ecological areas, and the resistance frequency of panicle blast is significantly lower than that of leaf blast (Fig. 1) (Wu et al, 2016, 2017). Therefore, there are few of broad-spectrum R genes that can effectively protect against both seedling and panicle blast.
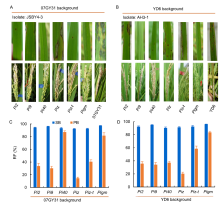 | Fig. 1. Comparison of leaf blast and panicle blast resistance among different alleles of Piz locus. A, Resistance reaction to leaf blast and panicle blast of different alleles from the Piz locusjaponica rice 07GY31 against the M. oryzae isolate JSBY4-3. B, Resistance reaction to leaf blast and panicle blast of different alleles from the Piz locus in the background of indica rice Yangdao 6 (YD6) against the isolate of M. oryzae (AH3-1). C, Resistance frequency (RF) to leaf blast and panicle blast of different alleles of the Piz locus in the background of japonica rice 07GY31. D, RF to leaf blast and panicle blast of different alleles of the Piz locus in the background of indica rice Yangdao 6. SB, Seedling blast; PB, Panicle blast. Data are Mean ± SD (n = 3). Blast resistant phenotypes were reproduced from Wu et al (2016, 2017). |
Gene pyramiding is generally considered an effective way to develop varieties with broad-spectrum and durable resistance. The rice variety Jefferson, with the gene combination Pik/Piz, has remained resistant since its first application in 1997 (McClung et al, 1997; Fjellstrom et al, 2004). Chen et al (2001) showed that resistance frequencies of monogenic lines with Pi1 and Pi2 are 92.45% and 89.65%, respectively, and those of polygene pyramiding lines (PPLs) withPi1andPi2 are as high as 98.04% against 715 isolates of M. oryzae. The additive effect of the monogenic lines broadens the resistance spectrum of the PPLs, resulting in an increase in the blast resistance. Similarly, pyramiding of Pi46 and Pi-ta enhances resistance compared to Pi46 and Pi-ta monogenic lines (Xiao et al, 2016). Yu et al (2013) confirmed pyramiding of two genes with different overlapping resistance spectra can improve the resistance of plants. However, the resistance effect of pyramiding lines does not comprise the simple accumulation of the resistance spectrum of target R genes. There is a significant interaction among the pyramided R genes, causing both positive and negative deviation (Tabien et al, 2000). Therefore, a random combination of two or more R genes can result a lower resistance effect of PPLs than the monogenic lines. International Rice Research Institute constructed a set of NILs carrying Pi1, Pi2 and Pi-ta, as well as PPLs carrying 2-3 R genes in the background of CO39. The results showed that the resistance of PPLPi2/Pi-ta is lower than that of the lines harboring only Pi2 (Hittalmani et al, 2000). Similarly, He et al (2001) found that the resistance of PPLPi1/Pi-ta is lower than that of the monogenic lines. In the background of japonica 07GY31, the resistance level of PPLPiz-t/Pi54 is significantly higher than that of monogenic lines harboring a single R gene. However, the resistance level of PPLPi9/Pi54 is significantly lower than that of monogenic lines harboring Pi9(Xiao et al, 2017). In the indica background of Yangdao 6, the resistance frequency of monogenic lines with Pi33 is slightly higher (70.8%) than that of the combination Pi33with Pish PPLPi33/Pish (69.1%) (Xiao et al, 2018). Recently, Wu et al (2019) evaluated the resistance effects of different alleles of Piz locus (Pigm, Pi40, Pi9, Pi2 and Piz) combined with other broad-spectrum R genes (such as Pi1, Pi33 and Pi54) systematically. They found that different gene combinations produce different interaction effects, in which most PPLs show no resistance comprising the simple accumulation of the resistance spectra of the target R genes. Among them, Pigm/Pi1, Pigm/Pi54 and Pigm/Pi33 are the most effective gene combination patterns, displaying stable broad-spectrum resistance under various conditions. Therefore, the combination of R genes directly affects the resistance level of the PPLs. Thus, to achieve broad-spectrum resistance to both leaf blast and panicle blast, resistance gene combination patterns must be assessed first in gene pyramiding breeding.
The development of molecular biology has brought rice breeding to the stage of combining biotechnology with conventional technology. In the last decade, molecular breeding technology, represented by molecular marker-assisted selection, has played an important role in the improvement of rice blast resistance in elite recipients. Many studies have been reported in this area, and some successful and representative examples are listed in Table 2. The donor R genes used in these molecular breeding studies are mainly alleles or tightly linked R genes from three loci, Piz, Pik and Pi-ta, including Pi2, Pi9, Pi40, Piz-t and Pigm of the Piz locus; Pi1 and Pi54 in the Pik locus, and Pi-ta from Pi-ta locus. Since there are so many identified R genes, why is molecular breeding practice only limited to few R genes? The reasons might be as follows: Firstly, the alleles or tightly linked R genes from these loci often exhibit relatively broad-spectrum resistance, especially in leaf blast resistance at the seedling stage. However, many R genes in other loci have a narrower resistance spectrum or less resistance effect, resulting in no obvious effect using other R genes in resistant improvement. Secondly, the cumbersome chain of linkage drag of some R genes produces negative effects on agronomic traits, which limits their utilization in breeding practice.
| Table 2 Successful examples of application of broad-spectrum resistance genes in rice breeding practice. |
An important prerequisite for molecular breeding improvement of rice blast resistance is to understand which R genes have been utilized in modern varieties and whether the resistance performance of these R genes is effective. Currently, the analysis of resistance genotypes in modern improved varieties uses linked markers or functional markers for cloned R genes. For example, Xiang et al (2018) analyzed distribution and use of rice blast resistant genes in the main cultivated rice varieties from Heilongjiang Province, China. The distribution frequencies (DFs) of Pi-ta, Pi5 and Pib are higher than those of other genes, reaching 31.37%, 29.41% and 18.62%, followed by Pi2, Pi-d2 and Pi-d3 with DFs of 9.80%, 1.96% and 1.96%, respectively. However, no Pi9is detected. Pi-ta, Pib, Pikm, Pi54 and Pi5 are detected in the core rice germplasms in Ningxia Province, China (Li Y D et al, 2019). Ma et al (2018) identified relatively high DFs of Pi5 and Pi54 in local varieties in Guizhou Province, China, at 32.35% and 30.86%, respectively. While the DFs of Pi9 and Pi2 are relatively lower, at 2.56% and 2.47%, respectively. Wu Y Y et al (2015) analyzed the distribution of cloned R genes in 277 main indicaandjaponica parental lines and showed that Pi5, Pi-d2, Pi-ta, Pia, Pib, Pb1, Pi54, Pish and Piz have relatively higher DFs (> 15%), whereas Pi2, Piz-t, Pi-d3, Pik and Pit have relatively lower DFs (< 10%), and Pi1, Pi9, Pi40 and Pigm have DFs of less than 2% and are found in local varieties, related wild species, or improved intermediate materials. Further analysis showed that some R genes are specifically distributed in the genomes of rice sub-species, for example, Pi5, Pit, Pi-d2 and Pi-d3 are mainly distributed in indica- type accessions, and Piz, Piz-t, Pb1, Pik and Pish are mainly harbored in japonica-type accessions, while Pi-ta, Pib, Pia and Pi54 are evenly distributed in both accessions. The above results provide us with a general understanding of the R genes used in the main rice parental lines in China. However, it should be noted that the judgment of the existence of the target R genes in the above studies are based on closely linked molecular markers or functional markers. Therefore, detection using molecular markers has a certain degree of correlation with R genes, but is more of an inference than a certainty, and cannot accurately reflect the presence of the target R gene. With the advances in third generation genome sequencing technology and the reduction of sequencing costs, it has become easier to quickly obtain high quality whole genome sequences.
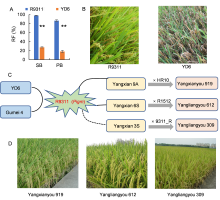
The existence of a defined resistant germplasm with an R target gene is an important prerequisite for breeding applications. Apart from the above- mentioned cloned R genes and some R genes used in modern improved varieties, many broad-spectrum genes, including Pigm, Pi9, Pi40, Pi1, Pi33 and Pi56, have not been utilized in modern varieties. These R genes are mainly distributed in local varieties or germplasms and might exhibit drag, causing poor agronomic traits and low yield. To promote the utilization of these genes in molecular breeding, it is necessary to overcome the linkage drag and create core resistant germplasms with excellent and comprehensive agronomic traits. Some explorations in this field have been made. For example, Pigm is a broad-spectrum R gene against both leaf and panicle blast (Wu et al, 2016, 2017). However, Pigmalso has the effect of reducing grain weight but increasing grain number (Deng et al, 2017). Although it can achieve yield balance, decrease in grain weight will lead to a reduction in rice yield and its marketability. Grain weight is a quantitative trait controlled by multiple genes, and therefore, it is affected by many other genes besides Pigm. In theory, individuals with no significant decrease in grain weight can be obtained by large-scale selection. Therefore, Wu et al (2016, 2017) used the elite restorer line Yangdao 6 (9311) as the recurrent parent, crossed it with the Pigm donor Gumei 4, and backcrossed the progeny continuously. A series of NILs have been obtained through foreground selection of target genes and large-scale screening of agronomic traits. The results of agronomic trait investigation showed that although the grain weight of some NILs is decreased, there are also some lines with similar grain weight and other elite agronomic traits to the recurrent parents (Fig. 2-A and B). Finally, core resistance germplasm carrying Pigm was selected and named as R9311. Using R9311 as the restorer line, two-line hybrid rice Yangliangyou 309 is bred. Moreover, using R9311 as the R gene donor parent, two-line cytoplasmic male sterility (CMS) line Yangxian 6S and three-line CMS line Yangxian 9A are bred, followed two-line hybrid rice Yangliangyou 612 and three-line hybrid rice Yangxianyou 919 (Fig. 2-C). These hybrid rice combinations performed well in regional trials and production tests (Fig. 2-D), and have also been approved by national certification. This serves as a successful example of the creation of core germplasm and molecular breeding for rice blast resistance.
Gene pyramiding helps to develop varieties with broad-spectrum and durable resistance to M. oryzae. Many studies have shown that resistance is significantly associated with the number of R genes, which means that the greater the number of R genes found in the accessions, the higher their resistance against M. oryzae (Wu Y Y et al, 2015; Li et al, 2019). However, the number of R genes is not the only factor affecting resistance. On one hand, as the number of R genes increases, the improvement in the resistance level would gradually slow down, which term as law of ‘ diminishing returns’ between the number of pyramided R genes and resistance (Xiao et al, 2018; Wu et al, 2019). On the other hand, as the number of pyramided R genes increases, in addition to increasing the workload, the linkage drag with unacceptable traits might also increase. Therefore, how to employ a few of these R genes to achieve broad-spectrum and durable resistance must be considered during rice blast resistance breeding (Yang et al, 2008). From the perspective of pyramided R genes and their corresponding resistance, the combination pattern of R genes is a key factor in determining the resistance level of different varieties against M. oryzae (Wu Y Y et al, 2015). Once different R genes are pyramided, some gene combinations will show positive interactions, while other gene combinations might exhibit negative interactions (Hittalmani et al, 2000; Xiao et al, 2017; Wu et al, 2019). Therefore, it is essential to screen and determine the combination pattern of R genes that exhibit broad- spectrum and durable resistance to promote the practical use of molecular breeding for blast resistance.
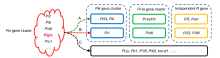
Several successful molecular breeding studies (Table 2) have indicated that a single R gene, such as Pi2, Pi9, Piz-t or Pi40 from the Piz locus shows broad-spectrum resistance to leaf and specifically exhibits resistance to panicle blast in some ecological regions. However, only Pigm shows broad-spectrum resistance to both leaf and panicle blast, and has an important practical value in breeding. Combining Pi2 with Pi1 from the Pik locus is the most successful example of the application of gene pyramiding, and there have also been reports about pyramiding with Pi5 and Pi-ta. Wu et al (2019) reported that Pigm exhibits high resistance to leaf and panicle blast after pyramiding with Pi1, Pi33 and Pi54, respectively. Moreover, they also found that the R genes of the alleles from the Piz locus exhibit excellent resistance after combining them with certain independently distributed R genes, such as Pi56 (unpublished data) and Pish (Xiao et al, 2018). Based on the distribution and utilization of cloned genes, we proposed that the broad-spectrum R genes of the Piz locus, especially Pigm, should be used as the backbone and core for gradual pyramiding of other R genes at three levels (Fig. 3). Firstly, pyramiding with Pi54 from the Pik locus, Pi-ta/Ptr from the Pi-ta locus, and the independently distributed R genesPi5 and Pish. These R genes have higher DFs, less linkage drag of poor agronomic traits, and are relatively easy to integrate. Secondly, pyramiding with Pi1from the Pik locus, Pi48 from the Pi-ta locus, and the independently distributed R genes Pi33 and Pi56. These R genes are generally distributed in local varieties or original germplasms and can be used in pyramiding breeding by creating core germplasms carrying target R genes and showing elite agronomic traits. Thirdly, on the basis of further clarifying the resistance effect of gene combinations, pyramiding could be performed using certain partial resistance genes, such as Pb1, Pi35, Pi63 and bsr-d1.
The development of genome sequencing technology has promoted the rapid identification and cloning of rice blast R genes and AVR genes, which has deepened understanding of the molecular mechanisms of rice blast fungus interaction and their co-evolution. It provides not only new genetic resources for rice blast resistance improvement, but also new technical ideas to regulate disease-resistance signaling pathways via genetic editing to achieve resistance improvement.
High-throughput whole genome sequence or target gene sequencing in the elite rice cultivars or core resistant germplasm will provide useful technological means for breeding selection. Since the cloning of Pi2, Pi9andPigm (Deng et al, 2017; Yang et al, 2019), functional single nucleotide polymorphism related to resistant genotypes can be used to design Kompetitive Allele-Specific PCR (KASP) markers and used in marker-assisted selection. Wang et al (2019) sequenced an important broad-spectrum blast resistant germplasm (Tetep) that is the donor of Pi5, and obtained a high-quality assembly, in which 455 nucleotide-binding site leucine-rich repeat (NLR) genes are annotated. Based on this, a few molecular markers have been designed to rapidly introduce clustered and paired NLRs in the Tetep genome to breed new resistant cultivars. With more donors of R genes being sequenced, the R genes confering a resistant genotype on elite rice cultivars or core resistant germplasms could be identified.
With the increase in genetic research into blast resistance, understanding of the partial resistance of non-race specificity, controlled by multiple quantitative trait loci (QTLs), has became a hot topic. Partial resistance is generally regarded as a quantitative trait. It does not prevent infection by M. oryzae but can reduce the proliferation of pathogens in the host and maintain a relatively low selection pressure on the population of M. oryzae, thus maintaining broad-spectrum and durable resistance (Niks et al, 2015). Since Wang et al (1994) first used restriction fragment length polymorphism markers to identify 19 QTLs controlling partial resistance in the durable resistance African upland rice cultivar Moroberekan, at least 500 QTLs for resistance to rice blast have been identified on the 12 chromosomes of rice to date (Li et al, 2019). In recent years, with the rapid development of molecular biology, a large number of partial resistance genes such as pi21 (Fukuoka et al, 2009), Pb1 (Hayashi et al, 2010), Pi35 (Fukuoka et al, 2014), Pi63 (Xu et al, 2014), bsr-d1 (Li et al, 2017), Bsr-k1 (Zhou et al, 2018) and Pid3-I1 (Inukai et al, 2019) have been cloned, suggesting that the rapid introduction of these R genes into rice varieties through molecular breeding is feasible (Pilet-Nayel et al, 2017). Fukuoka et al (2015) first reported that the four partial resistance genes, pi21, Pi34, qBR4-2 and qBR12-1, can be combined to improve durable resistance of rice. However, because of the small resistant effect of a single partial R gene, it must be combined with multiple partial resistance genes to obtain effective resistance. With the establishment of high-throughput molecular breeding methods, the creation of core germplasms harboring target partial resistance without linkage drag will become an important step in rice blast resistance improvement in the future.
In recent years, a series of important advances have been made in understanding the molecular mechanism of rice blast resistance. Significant progress has been achieved in cloning and identifying a number of key PTI and ETI signal regulation genes (Liu et al, 2014; Nasir et al, 2018), downstream signaling pathway related genes (Choi et al, 2015), and R proteins, especially downstream signaling molecules directly regulated by NBS-LRR proteins. However, compared with those 30 R genes that have been cloned, there has been less research on the downstream signaling molecules of R genes. Currently, three kinds of downstream signaling molecules that interact directly with R genes have been identified: OsRac1 signaling pathways downstream ofPit, Piaand Pi-d3 (Chen et al, 2010; Kawano et al, 2010; Wang et al, 2018; Zhou et al, 2019); the ARM repeats of OsPUB15 and Ptr(t) downstream of Pi-d2 and Pi-ta, respectively (Jia and Martin, 2008; Wang et al, 2015; Zhao et al, 2018); and transcription factor signaling pathways of WRKY45 and OsBIHD1, which act downstream of Pb1 and Pik-H4, respectively (Inoue et al, 2013; Liu et al, 2017). OsRac1 encoding a guanylate triphosphatase (GTPase), a member of the RhoGTPase family, is a key regulator of rice resistance to pathogens and it can participate in both PTI (Akamatsu et al, 2013) and ETI (Kawano et al, 2010). Zhou et al (2019) suggested that OsRac1 might be a common regulatory factor downstream of rice NBS-LRR proteins. Therefore, strengthening research into the interaction between OsRac1 and other R genes, especially the study of different broad-spectrum alleles from the Pizand Pik loci, should be promoted. At the same time, further identification of new signaling genes and analysis of the molecular regulatory mechanism of broad- spectrum resistance against M. oryzae are needed. OsERF922, an APETELA2/ethylene response factor (AP2/ERF) type transcription factor in rice, is rapidly and strongly induced by avirulent pathovars of M. oryzae. When the expression of OsERF922 was inhibited in RNAi transgenic lines, their blast resistance was enhanced (Liu et al, 2012). Similar research showed that mutant lines of OsERF922 edited by CRISPR/Cas9 show significantly higher resistance to M. oryzaecompared with the wild type (Xu et al, 2019). The expression levels of defense-related genes involved in signaling pathways of salicylic acid, jasmonic acid and ethylene metabolisms are upregulated in the mutant lines after inoculation of the physiological races of M. oryzae. Otherwise, some QTLs identified as necessary loci are required for resistance to rice panicle blast. Inoue et al (2017) mapped four QTLs that contribute to Pb1-mediated panicle blast resistance. In addition, a genome-wide association study of blast resistant loci or genes was used to identify novel R genes, and new Pik alleles, Pikxand Pif, respectively (Wang et al, 2014; Li C G et al, 2019). These identified genes not only provide new genetic resources for breeding broad-spectrum and durable rice cultivars, but also provide new strategies to improve resistance to rice blast.
This study was supported by the National Key Research and Development Program of China (Grant No. 2017YFD0100400), the Key Studying and Developing Project of Jiangsu Province for Modern Agriculture (Grant No. BE2018351), the Major Project of Jiangsu Province for Significant New Varieties Development (Grant No. PZCZ201702), the Jiangsu Key Laboratory of Crop Genomics and Molecular Breeding (Grant No. BM2018003), the National Natural Science Foundation of China (Grant No. 31971868), the National Modern Agricultural Industry Technology System Special Fund (Grant No. CARS-01-60), the ‘ 333’ Project of Jiangsu Province (Grant No. BRA2017163), the Key Studying and Developing Project of Yangzhou City for Modern Agriculture (Grant No. YZ2018048), and the Jiangsu Agricultural Science and Technology Innovation Fund [(Grant Nos. CX(18)1003) and CX(18)2022)], Open Research Fund of State Key Laboratory for Biology of Plant Diseases and Insect Pests (Grant No. SKLOF 201909), Opening Foundation of Key Laboratory of Plant Functional Genomics of the Ministry of Education (Grant No. ML201806), Fund of Institute of Agricultural Sciences for Lixiahe Region in Jiangsu (Grant No. SJ17201).
Managing Editor: Li Guan
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
| [107] |
|
| [108] |
|
| [109] |
|
| [110] |
|
| [111] |
|
| [112] |
|
| [113] |
|
| [114] |
|
| [115] |
|
| [116] |
|
| [117] |
|
| [118] |
|
| [119] |
|
| [120] |
|
| [121] |
|
| [122] |
|
| [123] |
|
| [124] |
|
| [125] |
|
| [126] |
|
| [127] |
|
| [128] |
|
| [129] |
|
| [130] |
|
| [131] |
|
| [132] |
|
| [133] |
|
| [134] |
|
| [135] |
|
| [136] |
|
| [137] |
|
| [138] |
|
| [139] |
|
| [140] |
|