Aromatic rice belongs to a small but important sub-group of rice, which is highly regarded for its excellent aroma and superior grain quality. Aromatic rice, especially Basmati- and Jasmine-type rice, is being traded at a high price in the local and global markets. Genetically, rice aroma is a phenotypical expression of spontaneous recessive mutations of the OsBadh2 gene (also known as fgr / badh2 / osbadh2 / os2APgene). These mutations inhibit the flow of γ-aminobutyraldehyde (GAB-ald) to γ-aminobutyric acid (GABA), and consequently, the accumulated GAB-ald is diverted to a potent flavour component 2-acetyl-1-pyrroline (2AP) by a non-enzymatic reaction with methylglyoxal. The natural incidence of non-functional osbadh2 mutation along with selection and nursing by the farmer from the ancient time makes rice aroma as a prominent natural gift. As GABA and methylglyoxal play significant roles in stress tolerance, and their biosynthesis is strictly regulated in rice plants, the accumulation of 2AP in aromatic rice depends on the interaction of various genetic and environmental factors, and its production may come at some costs of sacrificing tolerance. This review focused on some potential underlying genes in the 2AP and GABA biosynthesis pathways, and analyzed most aspects of aroma formation in rice, and summarized the molecular mechanism of aroma production together with its genetic and non-genetic influencing factors. The present review also stated approaches to produce high-quality aromatic rice via developing novel cultivars and with good agronomic knowledge-based practice.
Rice is the most important cereal crop and staple food consumed by more than half of the world’ s population (Wakte et al, 2017). Based on the presence of aroma, rice is classified as the aromatic and non-aromatic rice (Singh et al, 2000). Generally, non-aromatic rice varieties are high yielding, demonstrating good agronomic performance, and are highly adapted to the environmental condition and produced in all the rice-growing countries. Conversely, most of the aromatic rice varieties are low yielding, demonstrating inferior agronomic performance, highly prone to the environmental condition and are produced in few countries (Prodhan et al, 2017). Despite inferior performance, the aromatic rice is highly regarded for their excellent aroma and superior grain quality (Wakte et al, 2017). Phylogenetic analysis revealed that the aroma genes originate from the wild relatives (Oryza nivara and O. rufipogon), and the centre of origin is the foothills of the Himalayas in the Indian subcontinent from where it extends to different parts of the world (Pachauri et al, 2010). The diversity of aromatic rice varieties evolved over thousands of years by natural selection, continued by the local farmer’ s selection to suit their cultivation practices and preference, further improved by the plant breeders over a period of time (Prathepha, 2009; Pachauri et al, 2010). Hence, the aroma in rice can be considered as a natural phenomenon that has been nourished and continued by the selection process.
Genetically, aroma in rice is known to be controlled by a major gene osBadh2 (also recognized as fgr / badh2 / os2AP / osbadh2, LOC_Os08g0424500), which has been mutated, and expressed only at homozygous recessive conditions (Bradbury et al, 2005a; Bourgis et al, 2008; Shi et al, 2008). However, some aromatic rice varieties with good aroma do not contain the mutated alleles of the OsBadh2 gene, indicating the presence of other genes or alleles responsible for their aroma (Fitzgerald et al, 2008). All the genes responsible for aroma have not been fully identified (Huang et al, 2008; Kovach et al, 2009; Kaikavoosi et al, 2015).
Rice aroma quality also highly depends on the cultivation process along with environmental conditions like temperature, soil type, abiotic stress, water, CO2, light, salinity and shading (Itani et al, 2004; Mo et al, 2015). Basmati variety becomes more aromatic and expresses high-quality aroma when cultivated in the Punjab region of India and Pakistan, but it produces less aroma when grown in other regions or countries (Siddiq et al, 2012). In addition, its grain expresses excellent aroma when cultivated during relatively cool temperatures in the afternoon (25 º C-32 º C) and night (20 º C-25 º C) with humidity of about 70%-80% at the primordial and grain filling stages (Singh et al, 2000). Similarly, the Jasmine rice (KDML105) demonstrates higher aroma when grown in different rain-fed drought-prone areas in the north and north-eastern parts of Thailand containing dry and sandy soil than cultivated in other areas as well as in other countries (Yoshihashi et al, 2004). The aromatic rice varieties are usually susceptible to several diseases and pests, prone to most of abiotic stress, and highly responsive to photoperiodism (Nadaf et al, 2014; Mahajan et al, 2018; Feng et al, 2019). However, the inner mechanism of variation in aroma formation at different locations, molecular changes during various environmental stress conditions, and reasons behind the fluctuation of aroma under biotic and abiotic stress have not been investigated and explained completely.
A few papers have reviewed the biochemical, genetic, molecular basis and origin of aroma in rice (Champagne, 2008; Kovach et al, 2009; Vanavichit and Yoshihashi, 2010; Hashemi et al, 2013; Gaur et al, 2016; Wakte et al, 2017; Okpala et al, 2018; Somta et al, 2019), but molecular biological analysis of aroma biosynthesis and regulation are at the very preliminary stage, and explanation of the causes of the sensitivity of aromatic rice to the environmental changes is still lacking. Hence, this review aimed to provide deeper insight into the molecular mechanism of aroma production, its genetic and non-genetic influencing factors, and thus for developing and designing a suitable platform for the production of high-quality aromatic rice genotypes using modern plant breeding techniques.
Research on the genetics and molecular basis of aroma has been started in early 1970’ s when aroma detection methods from leaf samples were established, and further highlighted by mapping the single recessive gene (fgr) on chromosome 8 in rice. The molecular aspects of rice aroma can be explained by identifying important QTLs and mapping potential candidate genes for the aromatic compound.
A number of rice aroma QTLs have been identified on chromosomes 4, 8 and 12, while in Pusa 1121, at least three QTLs have been located on chromosomes 3, 4 and 8 (Lorieux et al, 1996; Amarawathi et al, 2008; Pachauri et al, 2014). Recently, three QTLs on chromosome 5 (one QTL) and chromosome 8 (two QTLs) were detected for rice grain aroma (Talukdar et al, 2017). However, until now only a few QTLs and associated markers have been confirmed as shown in Fig. 1.
 | Fig. 1. Integration of previously reported QTLs for aroma with respective candidate genes in rice. I, Luriex et al (1996); II, Singh et al (2007); III, Amaranthi et al (2008); IV, Pachuri et al (2014); V, Talukder et al (2017); Chr, Chromosome; Mb, Mega base; cM, Centimorgan. The map has been constructed using the Chromosome Map Tool of Oryzabase from http://viewer.shigen.info/oryzavw/maptool/MapTool.do; http://archive.gramene.org/qtl/ and http://archive.gramene.org/markers/ (information retrieved on 20 May, 2019). |
Development of an integrated physical and genetic map of rice has opened an era for performing efficient map-based gene cloning and associating candidate genes with important biological or agronomic traits (Chen et al, 2002). Based on the rice genome sequence information (IRGSP and Sasaki, 2005), the OsBadh2 gene (present on chromosome 8) is identified as a candidate gene for aroma, which is the most important aroma gene till now. Another locus (known as aro4-1) related to rice aroma has been identified on rice chromosome 4 (Lorieux et al, 1996; Amarawathi et al, 2008). During searching in the rice genome database for annotated function of genes present in the aro4-1 locus or QTL interval of chromosome 4, a gene for betaine aldehyde dehydrogenase 1 (OsBadh1; LOC_Os04g39020) is located in the same interval of 22 795 011-22 799 839 of the chromosome 4 pseudomolecule build 4 (AP008210.1) (IRGSP and Sasaki, 2005; Amarawathi et al, 2008). Hence, it was assumed that the OsBadh1 gene might be a candidate gene underlying in the aroma QTL aro4-1 due to the similar molecular function of the OsBadh2 gene. Furthermore, eight genes are co-localized as a cluster with the OsBadh1 gene on the aro4-1 locus, which might be an indication of having a significant role of this locus in the grain aroma expression (Pachauri et al, 2014). Another aroma QTL known as aro3-1 is identified in a new region of the rice genome that might be specific to the Basmati rice varieties (Amarawathi et al, 2008). Besides, one differentially expressed gene on aro3-1 locus on chromosome 3 is detected between markers CHR3_22 and CHR3_24, which is known to be a minor QTL for grain aroma sharing a complementary role with the other QTLs on chromosome 4 (aro4-1) (Pachauri et al, 2014). However, by the bulk segregation analysis approach, a single gene (LOC_Os03g21040) is constitutively down-regulated in both aromatic parents, as well as in aromatic bulk progeny. This gene belongs to the hydroxyproline-rich glycoprotein family, and is expressed under abiotic stress (Pachauri et al, 2014). Additionally, a major QTL between RM169 and RM430 has been identified on chromosome 5, which also harbours OsGlyI gene (13 094 299-13 098 871 bp), indicating an involvement of methylglyoxal to the rice aroma (Talukdar et al, 2017). The integrated information and positions of different potential QTLs along with candidate genes on different chromosomes related to rice aroma have been presented in Fig. 1 and explained in Table 1.
| Table 1 Description of some potential genes related to aroma in rice. |
From the integration mapping (Fig. 1) for analysis of candidate genes present within the previously reported QTLs, it can be assumed that OsBadh2 along with OsBadh1, OsGly and OsP5CSare responsible for aroma in rice (Bradbury et al, 2005a; Amarawathi et al, 2008; Yi et al, 2009; Fitzgerald et al, 2010; Pachauri et al, 2014; Talukdar et al, 2017). The OsBadh2 alleles possess insertions/deletions and single nucleotide polymorphisms, which result in loss of function and accumulation of 2-acetyl-1-pyrroline (2AP) in aromatic rice. However, some good aroma emitting rice varieties do not contain this allele, indicating the presence of other genes or alleles for rice aroma (Fitzgerald et al, 2008; Pachauri et al, 2010). It can be speculated that OsBadh1 (92% homology with OsBadh2) might also influence rice aroma (Shirasawa et al, 2006; Bourgis et al, 2008). The aromatic rice varieties show specific association with a BADH1 protein haplotype (PH2) having lysine144 to asparagine144 and lysine345 to glutamine345 substitutions due to the presences of SH1 and SH2 single nucleotide polymorphisms (SNPs) (Fig. 2). These two substitutions lead to a reduction in the substrate binding capacity of the BADH1 enzyme towards the γ -aminobutyraldehyde (GAB-ald) (Singh et al, 2010).
 | Fig. 2. Haplotypes of OsBadh1 gene in rice based on 15 single nucleotide polymorphisms (SNPs) with no missing data genotyped using Sequenom MassARRAY system. Protein haplotypes are based on three exonic SNPs (S6, S18 and S19). It was redrawn from Singh et al (2010). |
The OsBadh1 gene also functions in the oxidation of acetaldehyde in peroxisomes and the produced acetate is converted to acetyl-CoA to be utilized in the glyoxylate cycle in rice (Mitsuya et al, 2009). However, the involvement of OsBadh1 gene in rice aroma has not been studied as extensively as OsBadh2. Besides, another complementary minor QTL (aro3-1) for aroma trait has also been detected on chromosome 3 (Pachauri et al, 2014), and a potential candidate gene (OsGlyII, LOC_Os03g21460) is identified, which is involved in the detoxification of methylglyoxal. Contribution of methylglyoxal in elevating 2AP concentration in aromatic rice has been proven by reacting with Δ 1-pyrolline-5-carboxylate (P5C) non- enzymatically. Δ 1-pyrolline-5-carboxylate synthetase (P5CS) shows significantly higher activity in the aromatic rice calli than in non-aromatic rice (Huang et al, 2008). Two P5CS genes [OsP5CS1(LOC_Os05g38150) and OsP5CS2(LOC_Os01g62900)] have been identified in rice and overexpression of OsP5CS genes significantly increases proline content, P5CS activity and 2AP level in transgenic aromatic rice (Kaikavoosi et al, 2015). Hence, besides OsBadh2 gene, OsBadh1, OsGly(s) and OsP5CS(s) genes might be involved in the elevated level of 2AP as well as the aroma synthesis in rice.
A large number of compounds (more than 300 volatiles) have been recognized from different aromatic and non-aromatic rice, and determining which volatile compounds are responsible for the perceived aroma of rice is a difficult task (Yang et al, 2008). No single compound can be said to contribute a characteristic aroma except 2AP because it has the lowest odour thresholds and contains 1-pyrroline ring, in which the hydrogen at position 2 is replaced by an acetyl group with a methyl ketone group. The pyrroline ring makes the compound highly unstable and volatile (Buttery, 1982; Wakte et al, 2017). However, 2AP can be stabilized successfully for more than one year by making a derivative of 2AP using acids (Srinivas et al, 2006). In aromatic rice genotypes, 2AP can be detected in all parts of the plant except roots (Buttery et al, 1983; Maraval et al, 2010), whereas in non-aromatic rice, 2AP is also found at a much lower concentration (0.0015 mg/kg) that cannot be perceived easily (Widjaja et al, 1996). Until now, 2AP is considered as the main volatile compound for rice aroma, but initially, L-proline is identified as a precursor of 2AP in rice (Yoshihashi et al, 2002); later on, different views are raised regarding the origin of 2AP (Sakthivel et al, 2009). One of the prominent pathways might be the polyamine (an organic compound contains more than two amino groups) degradation pathway, which is the main 2AP synthesis pathway, and there might have some alternative pathways that also influence the 2AP concentration.
In this pathway, the polyamines (arginine, ornithine, spermidine, putrescine, etc.) are converted to GAB-ald (the immediate precursor of γ -aminobutyric acid, GABA), which spontaneously cyclises to Δ 1-pyrroline, an immediate precursor of 2AP and an important factor for regulating the rate of 2AP biosynthesis (Chen et al, 2008). By this pathway, the GAB-ald is converted to GABA by the functional BADH2 enzyme (coded by OsBadh2), which ultimately inhibits 2AP biosynthesis in non-aromatic rice. Conversely, the GAB-ald cannot be converted to GABA due to the non-functional badh2 enzyme (coded by osbadh2), which also results in GAB-ald accumulation, leading to the formation of 2AP in aromatic rice (Bradbury et al, 2008).
Another pathway can be the formation of P5C, an immediate precursor of proline synthesized from glutamate, which can react directly with methylglyoxal to form 2AP, and there might be no direct role of BADH2 enzyme for 2AP formation (Huang et al, 2008). However, the biosynthesis of 2AP depends on various enzymatic (gene dependent) and non-enzymatic (gene independent) pathways. The enzymatic pathways of 2AP synthesis are also involved in glycolysis and polyamine degradation path, while non-enzymatic pathway is involved in the direct production of 2AP.
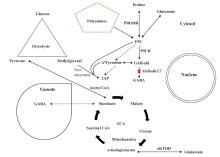
Based on the available information on the 2AP formation in prokaryotic and eukaryotic organisms, a proposed comprehensive diagram of 2AP biosynthesis pathway and its relation to other pathways in aromatic rice has been depicted in Fig. 3.
During biosynthesis of 2AP in aromatic rice, proline/ornithine/glutamate serves as the source of nitrogen, GAB-ald/Δ 1-pyrroline/1-pyrroline as a source of pyrroline ring, and 2-oxopropanal/methylglyoxal serves as a carbon source for 2AP formation (Romanczyk Jr et al, 1995; Huang et al, 2008). 2AP can be synthesized non-enzymatically at room temperature simply by stirring a mixture of P5C and methylglyoxal for 30 min (Huang et al, 2008), which is also reported in the aromatic vegetable soybean (Glycine max L.) (Wu et al, 2009).
Proline produced from glutamate, acting as a signalling molecule, accumulates more during stress condition and is converted to P5C by P5CS enzymes (P5CS1 & P5CS2). The produced P5C might react with methy- lglyoxal non-enzymatically or might be converted to Δ 1-pyrroline and eventually enhance 2AP concentration in aromatic rice. The formed Δ 1-pyrroline can also react with methylglyoxal non-enzymatically and be converted to 2AP in aromatic rice (Huang et al, 2008).
Methylglyoxal, produced through the glycolysis pathway, is mostly detoxified by the actions of glyoxalase enzymes (glyoxalase I, glyoxalase II and glyoxalase III) and the basal levels of methylglyoxal remain low in plants under normal growth conditions. However, during the stress conditions, the methylglyoxal can be accumulated at higher levels and the stress- induced methylglyoxal functions as a toxic molecule (inhibiting different developmental processes, including seed germination, photosynthesis and root growth). Hence, methylglyoxal may act as an important signalling molecule in regulating diverse events, such as cell proliferation and survival, control of the redox status of cells, and many other aspects of general metabolism and cellular homeostasis by transmitting and amplifying cellular signals and functions that promote adaptation of plants growing under adverse environmental conditions (Hoque et al, 2016). Down- regulation of glyceraldehyde-3-phosphate dehydrogenase C (GapC) and up-regulation of triose-phosphate isomerase (TPI) in aromatic vegetable soybean result in the accumulation of triose phosphate, thus leading to increases of methylglyoxal level and 2AP concentration (Wu et al, 2009). The presence of glyoxalase enzyme pathway, as well as the non-enzymatic reaction of methylglyoxal with P5C and Δ 1-pyrroline to form 2AP, might enough to speculate that 2AP is a generative volatile compound to detoxify methylglyoxal in rice plant.
Regulation of aroma in aromatic rice mainly depends on the environmental conditions that influence aroma quality and 2AP concentration, which has been tried to elucidate over the past few decades. Sometimes, stress during cultivation, such as drought (Yoshihashi et al, 2002) and salinity stress (Gay et al, 2010; Poonlaphdecha et al, 2012) led to higher 2AP content in rice grains (Table 2). Hence, rice aroma depends on both genetic and environmental factors, and aroma quality can be regulated by good management practices. In this review, the regulation or the reasons of fluctuation of aroma in aromatic rice were categorised into three categories, i.e. response to abiotic stress, response to temperature, and response to cultivation process.
| Table 2 Variation in 2-acetyl-1-phrroline (2AP) concentration under different environmental conditions. |
Aromatic rice is highly sensitive to abiotic stress, which reduce crop productivity and grain quality. The major abiotic stresses are salinity, drought, extreme temperature, submergence and ultraviolet irradiation (Cha-um et al, 2007). However, rice plants have evolved a number of protective mechanisms involving proline, methylglyoxal, GABA and calmodulin that act as signalling molecules and protective agents during stress conditions (Tester and Davenport, 2003; Bartels and Sunkar, 2005). GABA can be produced either by polyamine degradation pathway (functional OsBadh2 gene) or by GABA-shunt pathway (activity of OsGAD genes). It can be assumed that the GABA-shunt pathway becomes more active during stress conditions. The nature and severity of stress that are sensed through cellular changes in Ca2+ and/or H+ concentration, activate OsGAD genes to produce GABA by GABA-shunt pathway. GABA binds to the GABA-like receptor which releases Ca2+ from intracellular storage to increase Ca2+ accumulation in cytosol. The cytosolic Ca2+ amplifies the Ca2+/CaM complex and stress response signal, and induces stress response genes. The GABA-like receptors might also be involved in the acquisition of minerals that activate enzymes in stress-related metabolic pathways (Kinnersley and Turano, 2000). Recently, it has been shown that the non-aromatic and aromatic rice lines with specifically inhibited OsBadh1 and OsBadh2 genes are more susceptible to salt stress than wild-type with normal gene expression (Niu et al, 2008; Hasthanasombut et al, 2010, 2011). The concurrent increment of salt concentration and OsBadh1 gene transcript in the leaf tissue along with significant difference in the ability to produce mature seed in both non-aromatic and aromatic rice in the presence of salt, indicated the involvement of both OsBadh1 and OsBadh2 genes in salinity tolerance, and probably, their responses differ at different growth periods (He and Park, 2015). OsBadh1 might be active at the germination and seedling stages while OsBadh2 at the reproductive stage (He and Park, 2015; Hashemi et al, 2018). Moreover, an interaction between 2AP concentration and stress response is observed. For instance, drought stress during grain formation increases 2AP content (Yoshihashi et al, 2002) and salt stress during vegetative growth increases 2AP accumulation (Gay et al, 2010). One of the reasons of increasing 2AP content during stress conditions might be the emission of the starch-bound form of 2AP (requiring higher extraction temperature) together with the free form of 2AP (Yoshihashi et al, 2005). A common feature of environmental stress, as well as the changes and the survival mechanism of aromatic rice plants is depicted in Fig. 4.
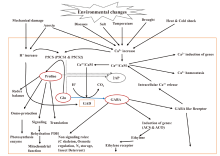 | Fig. 4. Roles of GABA and proline in plant stress responses and environmental changes. 2AP, 2-acetyl-1-phrroline; ACS, 1-aminocyclopropane-1-carboxylic acid synthase; ACO, 1-aminocyclopropane-1-carboxylic acid oxidase; CaM, Calmodulin; GABA, γ -aminobutyric acid; GAD, Glutamate decarboxylase; Glu, Glutamate; P5CS, Δ 1-pyrroline-5-carboxylate synthetase; PDH, Proline dehydrogenase. GABA and proline may function as a cellular barometer and transducer of environmental stress signals (Modified from Kinnersley and Turano, 2000). |
During stress conditions, the stimuli trigger influx of Ca2+ and/or H+ that activate OsP5CS and OsGAD genes to produce proteins for signal transduction regulation of mitogen-activated protein kinase (MAPK) pathway (Hashemi et al, 2018). These genes produce proline from polyamine degradation pathway and GABA via GABA-shunt pathway, respectively. Both proline and GABA act as signalling molecules to improve tolerance to stress. Stresses also induce the production of methylglyoxal via glycolysis pathway and increase the concentration of polyamines which has been reviewed in details (Shelp et al, 2012). However, methylglyoxal is a housekeeping signalling molecule present at a lower concentration but deleterious at a higher concentration. The elevated methylglyoxal can be converted to pyruvate by enzymatic reaction or 2AP by non-enzymatic reaction with Δ 1-pyrroline in aromatic rice. The methylglyoxal also can react with P5C non-enzymatically to produce 2AP. In aromatic rice, GABA can be produced only by the GABA-shunt pathway because the polyamines to GABA pathway remains inactive due to non- functional BADH enzyme.
Hence, the elite aromatic rice varieties are susceptible to abiotic and biotic stresses (Niu et al, 2008), and an association of aroma phenotype with salt susceptibility has been reported (Fitzgerald et al, 2010; Wijerathna et al, 2014). In addition, soil, temperature, relative humidity, moisture content and pH during flowering to maturity stages highly affect aroma quality and survival of aromatic rice plant by regulating glycolysis, 2AP and GABA-shut pathway.
Temperature highly influences the aroma quality in rice in all states including high (> 30 º C), low (< 20 º C) and optimum temperatures (20 º C to 30 º C). Aromatic rice produces more 2AP when grown at optimum temperature or optimum environmental or suitable growing conditions (Prodhan et al, 2017). It can be hypothesised that enzymes involved in the main 2AP biosynthesis pathway (polyamine degradation pathway or main 2AP synthesis pathway) remain more active during this situation. The evaporation rate of 2AP might be low, and methylglyoxal from glycolysis pathway signals for maintaining the physiological process and contributes to produce 2AP by reacting non-enzymatically with Δ 1-pyrroline. Under optimum temperature condition, the polyamines (glutamate, proline, ornithine, putrescine, spermidine, arginine, etc) are converted to Δ 1-pyrroline content which remains equilibrium to the GAB-ald concentration (Vanavichit and Yoshihashi, 2010). Besides, the proline produced from glutamate can be converted to GAB-ald by proline dehydrogenase and P5CR enzymes. Thereafter, GAB-ald can be converted to 2AP instead of GABA in aromatic rice due to the non-functional osbadh2 gene (Fig. 5).
GAB-ald is maintained in an equimolar ratio with Δ 1-pyrroline, and the GAB-ald levels appear to be an important factor regulating the rate of 2AP biosynthesis (Chen et al, 2008). However, increased expression of OsP5CR in aromatic varieties, as well as associated elevated concentration of its product, indicates that P5C the immediate precursor of proline synthesized from glutamate usually, reacts directly with methylglyoxal to form 2AP without direct role of BADH2 (Huang et al, 2008). However, all the rice-producing countries do not contain favourable condition during aromatic rice growth stage, hence aromatic rice do not express the same aroma when grown in different environments. Further investigation is required to reveal the involvement of responsible enzymes for aroma and environmental mitigation which will connect the genetic, molecular and chemical aspect of aromatic rice.
Cultural methods or cultivation practices greatly influence the aroma and flavour of aromatic rice. The organic management using chicken litter produces grains with low protein content having more aroma, while high nitrogen fertilizer application produces grains with high protein containing low aroma (Champagne et al, 2007; Champagne, 2008). Additional, supplementations of N, P, Ca, Mg, Mn and Zn increase 2AP content, though the highest content is recorded in addition of P (2-fold) compared to the other nutrients (Monggoot et al, 2014). The timing of field draining and harvesting of rice considering physiological maturity, moisture content, and meteorological conditions can influence the aroma quality of rice (Champagne et al, 2005). Aroma content decreases with the maturity and the best aroma (aroma score 3) is found at 20 d after 50% flowering. Low planting density and early harvesting can also improve aroma content (Goufo et al, 2010). The different irrigation modes and maintaining soil water potential at 0 to 25 (± 5) kPa can significantly increase free proline and aroma contents in Guixiangzhan and Meixiangzhan varieties (Wang et al, 2013). The inner mechanism of aroma formation and the causes of fluctuation in 2AP content due to cultivation management practices remain to be investigated.
There are several aromatic cultivars worldwide, however, only a few of them have become prestigious in the world market due to their excellent aroma and premier grain quality. The renowned Basmati-type or Jasmine-type aromatic rice express supreme grain quality when produced in certain regions of specific countries, yet they are susceptible to biotic and abiotic stress, and produce significantly less grain yield than non-aromatic varieties. Hence, aroma production by rice plants might come by some sort of sacrifice and adjustment to resistance and tolerance that need to explore deeply.
The aromatic rice is generally prone to diseases and possesses lower resistance. For example, Basmati rice is susceptible to blast, bacterial leaf blight, stem borer and white-backed planthopper. Jasmine rice is also susceptible to brown planthopper, blast and bacterial leaf blight. Both traditional Basmati rice and Jasmine rice are photosensitive and require short day length during the flowering stage, thus, the harvest season is limited to only one crop per annum (Nadaf et al, 2014; Mahajan et al, 2018). The reduced resistance might be due to the lack of resistance genes, lower level of GABA, and absence of functional OsBadh2 gene. Besides, the non-functional osbadh2 gene might also hamper the production of jasmonic acid in peroxisome which greatly participates in biotic resistance.
Most of the aromatic rice genotypes contain non-functional Badh gene homologues (osbadh1 and osbadh2), which are also related to the abiotic stress tolerance of rice genotypes that have been described and reviewed in details (Hashemi et al, 2018). In addition, several truncated or recombinant transcripts of OsBadh1 and OsBadh2 genes due to unusual post-transcriptional process have been found in rice, which result in the insertion of exogenous gene sequences, deletion or elimination of the start codon, loss of a functional domain, and introduction of a premature termination codon (Niu et al, 2007). The OsBadh1 gene transcript exhibits a consistent increase in response to salt treatment in both aromatic and non-aromatic rice varieties, but the osbadh2 gene transcript levels did not increase in salt treatment (Fitzgerald et al, 2008). RNAi analysis of the transgenic non-aromatic rice with an inhibited expression of the Badh2 gene indicates that the badh2 gene expresses aroma in rice but reduces the ability of salt stress tolerance, though the OsBadh2 gene contributes to salt tolerance in rice (Niu et al, 2008; Fitzgerald et al, 2010). In an experiment, the aromatic rice lines produced a few mature seeds compared to non-aromatic rice lines when exposed to 17 and 22 mmol/L NaCl stress, and the mature seed production decreases by 92.0% to 96.5% compared to the non-aromatic rice lines (Fitzgerald et al, 2010). Hence, it is evident that both BADH homologues have a role in salt tolerance and producing mature seeds, but in aromatic rice, this BADH protein needs to be inactive, which is a phenomenon of sacrificing stress tolerance.
Polyamines, especially diamine putrescine and triamine spermidine, which are essential for several biological activities as well as responses to biotic and abiotic stresses, are utilized for 2AP formation in aromatic rice. The concentration of polyamines becomes lower in aromatic rice due to 2AP generation, which also might be a cause of reduced ability to stress tolerance. Besides, proline which is a stress-responsive molecule, becomes lower due to the conversion of 2AP in aromatic rice. The conversion of proline to 2AP occurs in stems and leaves before translocating to grains where such mechanism has already occurred in aromatic rice. This type of proline reformation might make them susceptible to environmental changes (Mo et al, 2016).
Since aromatic rice is a non-accumulator of glycine betaine, and GABA cannot be produced from GAB- ald due to non-functional BADH enzyme (Bradbury, 2009), it might decrease salt tolerance, produce low yield, and render susceptibility to abiotic and biotic stresses. Hence, the production of aroma by aromatic rice is a consequence of reduced adaptability and tolerance, which is still a challenge for the researchers worldwide.
Aroma is one of the most important grain quality traits of aromatic rice, and the demand and consumption of high-quality aromatic rice are increasing day by day and would be continued in the future. Hence, production of new aromatic rice varieties and continuation of research on aroma genes are extremely important and require intensive attention. The rapid development of rice functional genomics, modern molecular and biotechnological techniques can clear the genetic basis, as well as function and regulation of aroma genes in rice. These tremendous progresses in aroma research attract more scientists from diverse area of genetics, breeding and plant science. Hence, the practical and hypothetical ways to improve aroma quality are explained as below.
Aromatic rice grain quality can be improved using existing aromatic genotypes by backcrossing, mutation breeding or conventional cross-breeding. However, it is time-consuming and laborious to cultivate new varieties (Shan et al, 2015). Since aroma is controlled by a recessive gene, the osbadh2 mutant gene need to be incorporated into the existing elite varieties through hybridization and backcrossing, then screening the individuals in the offspring population. Research has been conducted on the cultivation of high-quality, high-yielding, multi-resistant aromatic rice varieties, as well as screening a series of aromatic rice restorer lines, sterile lines, and new hybrid combinations (Bao et al, 2007). With the development of molecular markers, especially the functional markers of osbadh2gene, molecular marker-assisted selection has been widely used in rice genetics and breeding of aromatic rice, which has greatly accelerated the process of screening and breeding of new aromatic rice varieties, and many varieties of aromatic rice have been approved and widely used in production (Peng et al, 2018).
The molecular markers closely linked to the fgr gene have been identified (Jin et al, 2003), a series of specific primers (functional markers) have been designed for the interior of the OsBadh2 gene (Bradbury et al, 2005b; He and Park, 2015), and transgenic technology has been developed for use in the creation of new aromatic rice varieties (Chen et al, 2012). The resistance genes or desired genes can be transferred into prominent aromatic rice genotypes, which will facilitate to overcome the limitation of existing aromatic rice varieties.
Non-aromatic rice which is highly adaptive, tolerant to stress and grown in all rice-producing countries, can be utilized to produce aromatic rice by manipulating the aroma genes. Any insertion, deletion or replacement of OsBadh2 gene will lead to premature termination codon or encoded amino acid change or even non-coding corresponding Badh2 protein in rice, which can eventually make non-aromatic rice to aroma producing rice (Bradbury et al, 2005a; Shi et al, 2008; Kovach et al, 2009). Therefore, the loss function of OsBadh2 gene can promote the synthesis and accumulation of 2AP, and it is conceivable that any mutation that causes the loss of OsBadh2 gene function will lead to the emergence of new aroma gene(s).
The RNAi mediated OsBadh2 gene silencing technique and artificial microRNA-induced down-regulation can be utilized to transform non-aromatic into aromatic rice varieties (Chen et al, 2012; Khandagale et al, 2017). The RNAi technique can not completely inhibit the expression of OsBadh2 gene, and a large number of transgenic progenies need to be screened. This technique is also facing the risk assessment of late transgenic and strict supervision. Besides, the certification procedure for the new transgenic rice using RNAi is still under consideration (Peng et al, 2018).
Using transcription activator-like effector nucleases (TALEN) technology, any non-aromatic rice varieties can be transformed into aromatic rice varieties, and the TALEN technology can also be used to create a genetically homozygous mutant of aromatic rice plants (Shan et al, 2013, 2015; Birla et al, 2017).
Recently, the clustered regularly interspaced short palindromic repeat-associated protein 9 (CRISPR/Cas9) system has been utilized successfully to produce aromatic rice from non-aromatic rice variety (Zhonghua 11) by Shao et al (2017). Now, the CRISPR/Cas9 tool can be decorated with all genome editing capabilities (e.g., knock-in, knock-out, knock-down, and expression activation), which has tremendous untapped potential, and an ever-expanding genetic toolbox for plant biologists to investigate functional genomics has been formed, which is helpful for breeders to manipulate important genes in the genomes of important crops. The ability to target multiple genes via multiplexed genome editing strategies can facilitate pathway-level research to engineer complex multigenic grain quality attributes in rice including rice aroma. The rapid shift of research toward the utilization of CRISPR/Cas9 system for targeted mutagenesis can be a promising approach for overcoming barriers in the breeding for improving aroma quality in rice (Fiaz et al, 2019).
Though modern genome editing techniques have utmost potentiality, some strategies need to be considered during designing experiment to overcome the limitations and improve aromatic rice production. Firstly, the OsGAD gene(s) of the GABA-shunt pathway can be manipulated for higher GABA content that would facilitate stress tolerance and 2AP production. The removal of a limited C-terminal region of OsGAD2 gene has been found to contribute directly to drastic enhancement of the glutamate decarboxylase enzyme activity (Akama and Takaiwa, 2007). Transgenic tobacco plants expressing a mutant osgad that lacks the auto-inhibitory CaM binding domain exhibit higher GABA levels, lower glutamate levels, and less stem elongation (Baum et al, 1996; Akama and Takaiwa, 2007). By manipulating OsGAD gene(s), the GABA content will be increased, which will enhance 2AP production and adaptability to the environmental changes. Secondly, auto-inhibitory properties of P5CS enzyme can be removed for more proline production. Proline content plays diverse roles in plant development and adaptation, and it can be elevated by increasing the expression levels of proline rate-limiting genes in transgenic rice plants or by removal of feedback inhibition properties of γ -glutamyl kinase of P5CS enzyme (Hong et al, 2000). The feedback regulation of P5CS is usually lost or hamper in plants under stress conditions. By manipulating OsP5CS genes, the proline content would be increased, which will enhance 2AP production and adaptability to the environmental changes. However, removal of the controlling mechanism for proline biosynthesis might affect other qualities of aromatic rice, which needs to be considered during designing the research. Thirdly, manipulation of the Gly genes in glyoxalase pathway for higher methylglyoxal production. Methylglyoxal is known to be a precursor of 2AP synthesis in aromatic rice as well as in other organisms (Phillips and Thornalley, 1993). The down- regulation of GapC gene and up-regulation of TPI gene result in the accumulation of triose phosphate and increase in methylglyoxal levels that consequently increase 2AP in the aromatic vegetable soybean (Wu et al, 2009). The engineering of OsGly genes can elevate the methylglyoxal content. This boosted methylglyoxal content can be detoxified non- enzymatically by reacting with P5C or Δ 1-pyrroline that would ultimately increase 2AP content and trigger the stress-responsive elements. Finally, precise mutations (InDels and SNPs) in the BADH genes (OsBadh1 and OsBadh2) can induce complete inactivation of BADH enzyme, which would facilitate higher 2AP content in rice. However, the choice of technology and the way to improve the aroma quality and plant adaptability depend on the breeder’ s choice as well as the consideration of breeding program.
The authors are grateful to the Institute of Crop Science, College of Agriculture and Biotechnology, Zhejiang University, China for providing postdoctoral fellowship, facilities and supports during this research.
(Managing Editor: Li Guan)
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|