
Rice Science ›› 2021, Vol. 28 ›› Issue (6): 529-531.DOI: 10.1016/j.rsci.2021.09.002
• Letter • Previous Articles Next Articles
Cong Liu1,#, Dongying Tang1,#, Zhengkun Zhou1,#, Hui Zeng1,#, Xiaochun Hu2, Yanning Tan3, Peng Qin2, Yong Deng1, Jicai Wu1, Yan Wang1, Yuanzhu Yang2, Dingyang Yuan3, Xuanming Liu1( ), Jianzhong Lin1(
), Jianzhong Lin1( )
)
Received:2020-12-23
Accepted:2021-07-09
Online:2021-11-28
Published:2021-11-28
About author:#These authors contributed equally to this work
Cong Liu, Dongying Tang, Zhengkun Zhou, Hui Zeng, Xiaochun Hu, Yanning Tan, Peng Qin, Yong Deng, Jicai Wu, Yan Wang, Yuanzhu Yang, Dingyang Yuan, Xuanming Liu, Jianzhong Lin. Efficient Transformation of indica Rice Mediated by Agrobacterium and Generation of NcGDH Transgenic Genic Male-Sterile Rice with High Nitrogen Use Efficiency[J]. Rice Science, 2021, 28(6): 529-531.
Add to citation manager EndNote|Ris|BibTeX
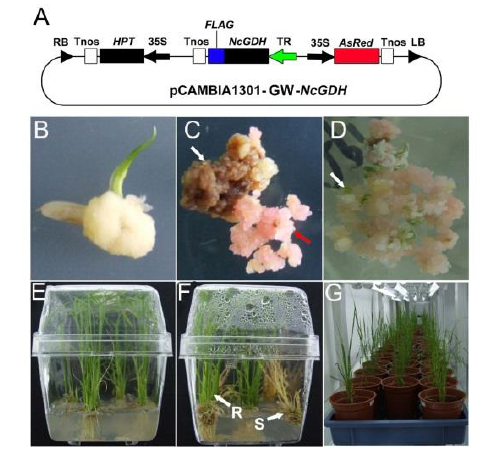
Fig. S1. Schematic diagram of plant expression vector and transformation procedure.A, Schematic diagram of pCAMBIA1301-GW-NcGDH expression. RB, Right border; Tnos, Terminator of nopaline synthase gene (nos); HPT, Hygromycin resistance gene; 35S, CaMV 35S promoter; FLAG, FLAG tag sequence; NcGDH, NADP(H)-GDH gene derived from Neurospora crassa; TR, Root-specific expression promoter TobRB7 derived from tobacco; AsRed, Red ?uorescence protein gene; LB, Left border. B, Induction of callus from a seed of indica rice Xiangling 628S. C, Hygromycin-resistant screen of the calli co-cultured with the Agrobacterium (EHA105 carrying pCAMBIA1301-GW-NcGDH vector). The white arrow indicates the non-transformed calli, and the red arrow indicates the transformed calli. D, Differentiation of shoots from the calli with hygromycin resistance and red fluorescence. The white arrow indicates the regenerated bud points. E, Differentiation of roots from the shoots regenerated from calli with red fluorescence. F, Differentiation of roots from the shoots regenerated from calli with hygromycin resistance but without red fluorescence. R, Hygromycin-resistant plantlet; S, Hygromycin-sensitive plantlet. G, Regenerated plantlets grown in a greenhouse for acclimatization.
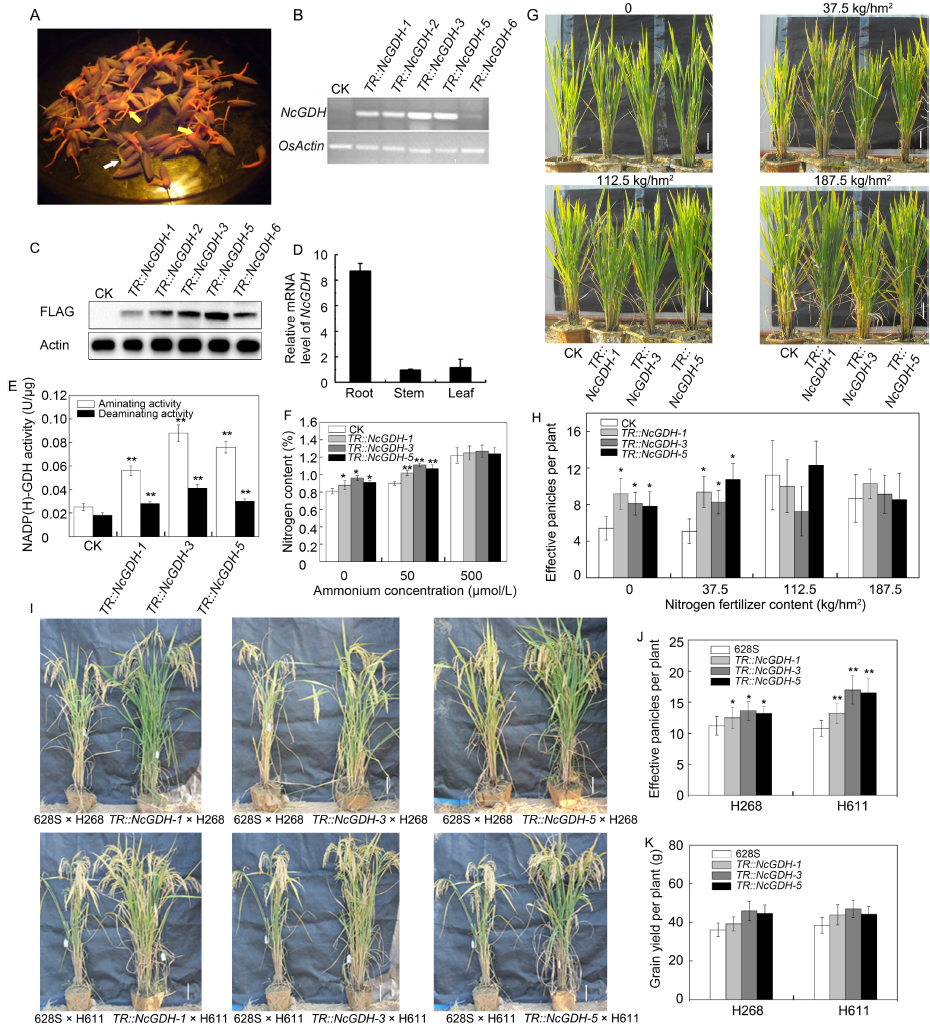
Fig. 1. Identification and phenotypic analysis of NcGDH transgenic 628S.A, Red fluorescence emitted from germinated seeds of NcGDH transgenic 628S (T1). The yellow and white arrows indicate germinated seeds with and without red fluorescence, respectively. B, Semi-quantitative PCR analysis of NcGDH expression level in different NcGDH transgenic lines and control plants (CK). C, Western blotting analysis of NcGDH transgenic lines and CK. D, Expression pattern analysis of NcGDH in transgenic lines by qRT-PCR. E, NADP(H)-GDH activities in the roots of CK and NcGDH transgenic lines. F, Nitrogen contents of CK and NcGDH transgenic lines. G, Phenotypes of CK and NcGDH transgenic lines grown in the field under different nitrogen fertilizer conditions. Urea was used as nitrogen fertilizer. H, Effective panicles per plant of CK and NcGDH transgenic lines under different nitrogen fertilizer (urea) conditions. I, Phenotypic comparison of hybrid rice generated by CK and NcGDH transgenic lines grown under low nitrogen conditions at the maturity stage. J and K, Effective panicles per plant (J) and grain yield per plant (K) of different hybrid rice lines. 628S, TR::NcGDH-1, TR::NcGDH-3 and TR::NcGDH-5 were used as the female parents; H268 and H611 were used as the male parents. In B, C and E-H, CK represents the negative control (non-transgenic 628S) plants. OsActin was used as an internal reference in B-D; Scale bars in G and I are 10 cm. Data in D-F, H, J and K are presented as Mean ± SD (n = 3). * and **, P ≤ 0.05 and P ≤ 0.01 by the Student's t-test, respectively.
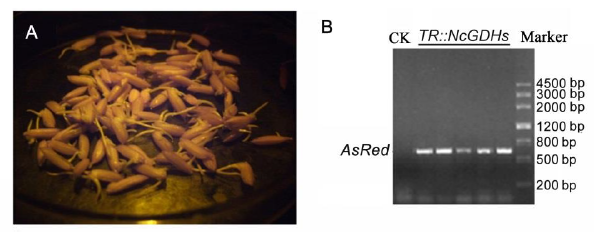
Fig. S2. Identification of NcGDH transgenic 628S.A, Red fluorescence emitted from germinated seeds of 628S. B, PCR analysis of AsRed in NcGDH transgenic lines and control plants. CK, Negative control (non-transgenic 628S) plants.
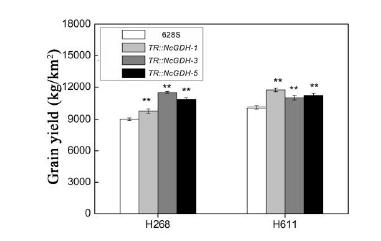
Fig. S3. Grain yield of different hybrid rice lines.Data are presented as Mean ± SD (n = 40). * and **, P ≤ 0.05 and P ≤ 0.01 by the Student’s t-test, respectively. H268 and H611 were used as male parents.
| Gene name | Primer sequence (5′-3′) | Purpose |
|---|---|---|
| NcGDH | F: ATGTCTAACCTTCCCTCTGAGCCCG | Gene cloning |
| R: TTAGTTCTTGGACCACCAGTCACCC | ||
| OsActin1 | F: CGGAGCGTGGTTACTCATTCA | Semi-quantitative PCR |
| R: ATCCTCCAATCCAGACACTGTACTTC | ||
| NcGDH | F: GAGCGTGTCATTCAGTTCCGTGTT | Semi-quantitative PCR |
| R: TGAGCTTGAGGGCGGCGTAC | ||
| AsRed | F: CACCATGGCCTCTTTGCTGAAG | PCR analysis |
| R: CCTCGTACTGCTTGTAGCACT | ||
| NcGDH | F: ACTGGCGAGTCCGGCATCAC | Real-time quantitative PCR |
| R: TCTGGGTAGCGCAAGGAAGAGC | ||
| OsActin1 | F: AACATCGTTCTCAGTGGTGGTA | Real-time quantitative PCR |
| R: GGAGGACGGCGATAACAG |
Table S1. Primers used in the study.
| Gene name | Primer sequence (5′-3′) | Purpose |
|---|---|---|
| NcGDH | F: ATGTCTAACCTTCCCTCTGAGCCCG | Gene cloning |
| R: TTAGTTCTTGGACCACCAGTCACCC | ||
| OsActin1 | F: CGGAGCGTGGTTACTCATTCA | Semi-quantitative PCR |
| R: ATCCTCCAATCCAGACACTGTACTTC | ||
| NcGDH | F: GAGCGTGTCATTCAGTTCCGTGTT | Semi-quantitative PCR |
| R: TGAGCTTGAGGGCGGCGTAC | ||
| AsRed | F: CACCATGGCCTCTTTGCTGAAG | PCR analysis |
| R: CCTCGTACTGCTTGTAGCACT | ||
| NcGDH | F: ACTGGCGAGTCCGGCATCAC | Real-time quantitative PCR |
| R: TCTGGGTAGCGCAAGGAAGAGC | ||
| OsActin1 | F: AACATCGTTCTCAGTGGTGGTA | Real-time quantitative PCR |
| R: GGAGGACGGCGATAACAG |
| Component | Amount for 1 L medium | ||
|---|---|---|---|
| MS medium | |||
| Macronutrient components (20×) | |||
| KNO3 | 38 g | ||
| NH4NO3 | 33 g | ||
| MgSO4·7H2O | 7.4 g | ||
| CaCL2·2H2O | 8.8 g | ||
| KH2PO4 | 3.4 g | ||
| Micronutrient components (1 000×) | |||
| MnSO4·4H2O | 22.3 g | ||
| ZnSO4·7H2O | 8.6 g | ||
| H3BO3 | 6.2 g | ||
| Copper and cobalt components (2 000×) | |||
| CuSO4·5H2O | 0.05 g | ||
| CoCL2·6H2O | 0.05 g | ||
| KI | 1.7 g | ||
| Na2MoO4·2H2O | 0.5 g | ||
| Ferric salt components (200×) | |||
| FeSO4·7H2O | 5.57 g | ||
| Na-EDTA·2H2O | 7.45 g | ||
| Organic components (200×) | |||
| Glycine | 0.4 g | ||
| Thiamine | 0.02 g | ||
| Pyridoxine | 0.1 g | ||
| Inositol | 20 g | ||
| Nicotinic acid | 0.1 g | ||
| AAM medium | |||
| Macronutrient components (20×) | |||
| KCl | 60 g | ||
| MgSO4·7H2O | 5 g | ||
| CaCL2·2H2O | 3 g | ||
| NaH2PO4·2H2O | 3 g | ||
| Micronutrient components (200×) | |||
| MnSO4·4H2O | 2 g | ||
| ZnSO4·7H2O | 0.4 g | ||
| H3BO3 | 0.6 g | ||
| Copper and cobalt components (2 000×) | |||
| CuSO4·5H2O | 0.05 g | ||
| CoCL2·6H2O | 0.05 g | ||
| KI | 1.5 g | ||
| Na2MoO4·2H2O | 0.5 g | ||
| Ferric salt components (200×) | |||
| FeSO4·7H2O | 5.57 g | ||
| Na-EDTA·2H2O | 7.45 g | ||
| Organic components (100×) | |||
| Glycine | 0.75 g | ||
| Thiamine | 1 g | ||
| Pyridoxine | 0.1 g | ||
| Inositol | 10 g | ||
| Nicotinic acid | 0.1 g | ||
| N6 or B5 medium | |||
| N6 macronutrient components (20×) | |||
| KNO3 | 56.6 g | ||
| (NH4)2SO4 | 9.26 g | ||
| MgSO4·7H2O | 3.7 g | ||
| KH2PO4 | 8 g | ||
| CaCL2·2H2O | 3.32 g | ||
| B5 micronutrient components (200×) | |||
| MnSO4·4H2O | 0.88 g | ||
| ZnSO4·7H2O | 0.3 g | ||
| H3BO3 | 0.32 g | ||
| Copper and cobalt components (2 000×) | |||
| CuSO4·5H2O | 0.05 g | ||
| CoCL2·6H2O | 0.05 g | ||
| KI | 1.7 g | ||
| Na2MoO4·2H2O | 0.5 g | ||
| Ferric salts components (200×) | |||
| FeSO4·7H2O | 5.57 g | ||
| Na-EDTA·2H2O | 7.45 g | ||
| NB organic components (200×) | |||
| Glycine | 0.4 g | ||
| Thiamine | 0.2 g | ||
| Pyridoxine | 0.1 g | ||
| Inositol | 20 g | ||
| Nicotinic acid | 0.1 g | ||
| D3 organic component (200×) | |||
| Glycine | 0.2 g | ||
| VB1 | 0.1 g | ||
| Thiamine | 0.1 g | ||
| Pyridoxine | 10 g | ||
| Nicotinic acid | 0.1 g | ||
| plant hormones and acetosyringone | |||
| National Aeronautic Association (NAA) | 0.2 g | ||
| 3-Indoleacetic acid (IAA) a | 0.2 g | ||
| Kinetin (KT) | 0.2 g | ||
| 6-Benzylaminopurine (6-BA) | 0.2 g | ||
| 2,4-Dichlorophenoxyacetic acid (2,4-D) | 0.2 g | ||
| Acetosyringone (AS) a | 200 mol | ||
| Induction medium | |||
| N6 macronutrient components (20×) | 50 mL | ||
| B5 micronutrient components (200×) | 5 mL | ||
| Copper and cobalt components (2 000×) | 0.5 mL | ||
| NB organic components (200×) | 5 mL | ||
| Ferric salts components (200×) | 5 mL | ||
| 2,4-D (0.2 mg/mL) | 12.5 mL | ||
| Casein hydrolysate | 0.3 g | ||
| Proline | 2.9 g | ||
| Sucrose | 30 g | ||
| PhytagelTM | 4 g | ||
| AAM medium | |||
| AAM macronutrient components (20×) | 50 mL | ||
| B5 micronutrient components (200×) | 5 mL | ||
| AAM organic components (100×) | 5 mL | ||
| Ferric salts components (200×) | 10 mL | ||
| AAM copper and cobalt components (2 000×) | 0.5 mL | ||
| Casein hydrolysate | 0.5 g | ||
| Glucose | 36 g | ||
| Sucrose | 68.5 g | ||
| L-Arginine | 0.18 g | ||
| L-Glutamine | 0.9 g | ||
| L-Asparagine | 0.3 g | ||
| AS (200 mmol/L) a | 0.5 mL | ||
| Co-culture medium | |||
| N6 macronutrient components (20×) | 50 mL | ||
| B5 micronutrient components (200×) | 5 mL | ||
| Copper and cobalt components (2 000×) | 0.5 mL | ||
| NB organic components (200×) | 5 mL | ||
| Ferric salt components (200×) | 5 mL | ||
| 2,4-D (0.2 mg/mL) | 12.5 mL | ||
| Casein hydrolysate | 0.3 g | ||
| Proline | 0.5 g | ||
| Glutamine | 0.5 g | ||
| Glucose | 10 g | ||
| Sucrose | 30 g | ||
| PhytagelTM | 4 g | ||
| AS (200 mmol/L) | 0.5 mL | ||
| Selection medium | |||
| MS macronutrient components (20×) | 50 mL | ||
| B5 micronutrient components (200×) | 50 mL | ||
| Copper and cobalt components (2 000×) | 5 mL | ||
| D3 organic components (100×) | 10 mL | ||
| Ferric salts components (200×) | 7.5 mL | ||
| 2,4-D (0.2 mg/mL) | 12.5 mL | ||
| Casein hydrolysate | 0.3 g | ||
| L-Glutamine | 0.5 g | ||
| Maltose | 30 g | ||
| PhytagelTM | 4 g | ||
| Hygromycin (50 mg/mL) | 0.6 mL | ||
| Carbenicillin (200 mg/mL) | 2 mL | ||
| Differentiation media | |||
| N6 macronutrient components (20×) | 50 mL | ||
| B5 micronutrient components (200×) | 50 mL | ||
| Copper and cobalt components (2 000×) | 5 mL | ||
| D3 organic components (100×) | 10 mL | ||
| Ferric salts components (200×) | 10 mL | ||
| Proline | 0.5 g | ||
| Casein hydrolysate | 0.8 g | ||
| L-Glutamine | 0.5 g | ||
| Maltose | 30 g | ||
| 6-BA (0.2 mg/mL) | 10 mL | ||
| KT (0.2 mg/mL) | 10 mL | ||
| NAA (0.2 mg/mL) | 1 mL | ||
| PhytagelTM | 4 g | ||
| IAA (0.2 mg/mL) | 1 mL | ||
| Rooting media | |||
| MS macronutrient components (20×) | 50 mL | ||
| MS micronutrient components (1,000×) | 1 mL | ||
| Copper and cobalt components (2 000×) | 0.5 mL | ||
| MS organic components (200×) | 5 mL | ||
| Ferric salts components (200×) | 5 mL | ||
| Sucrose | 30 g | ||
| Casein hydrolysate | 1 g | ||
| NAA (0.2 mg/mL) | 2.5 mL | ||
| PhytagelTM | 4 g | ||
| IAA (0.2 mg/mL) | 2.5 mL | ||
Table S2. Medium components used for indica rice transformation.
| Component | Amount for 1 L medium | ||
|---|---|---|---|
| MS medium | |||
| Macronutrient components (20×) | |||
| KNO3 | 38 g | ||
| NH4NO3 | 33 g | ||
| MgSO4·7H2O | 7.4 g | ||
| CaCL2·2H2O | 8.8 g | ||
| KH2PO4 | 3.4 g | ||
| Micronutrient components (1 000×) | |||
| MnSO4·4H2O | 22.3 g | ||
| ZnSO4·7H2O | 8.6 g | ||
| H3BO3 | 6.2 g | ||
| Copper and cobalt components (2 000×) | |||
| CuSO4·5H2O | 0.05 g | ||
| CoCL2·6H2O | 0.05 g | ||
| KI | 1.7 g | ||
| Na2MoO4·2H2O | 0.5 g | ||
| Ferric salt components (200×) | |||
| FeSO4·7H2O | 5.57 g | ||
| Na-EDTA·2H2O | 7.45 g | ||
| Organic components (200×) | |||
| Glycine | 0.4 g | ||
| Thiamine | 0.02 g | ||
| Pyridoxine | 0.1 g | ||
| Inositol | 20 g | ||
| Nicotinic acid | 0.1 g | ||
| AAM medium | |||
| Macronutrient components (20×) | |||
| KCl | 60 g | ||
| MgSO4·7H2O | 5 g | ||
| CaCL2·2H2O | 3 g | ||
| NaH2PO4·2H2O | 3 g | ||
| Micronutrient components (200×) | |||
| MnSO4·4H2O | 2 g | ||
| ZnSO4·7H2O | 0.4 g | ||
| H3BO3 | 0.6 g | ||
| Copper and cobalt components (2 000×) | |||
| CuSO4·5H2O | 0.05 g | ||
| CoCL2·6H2O | 0.05 g | ||
| KI | 1.5 g | ||
| Na2MoO4·2H2O | 0.5 g | ||
| Ferric salt components (200×) | |||
| FeSO4·7H2O | 5.57 g | ||
| Na-EDTA·2H2O | 7.45 g | ||
| Organic components (100×) | |||
| Glycine | 0.75 g | ||
| Thiamine | 1 g | ||
| Pyridoxine | 0.1 g | ||
| Inositol | 10 g | ||
| Nicotinic acid | 0.1 g | ||
| N6 or B5 medium | |||
| N6 macronutrient components (20×) | |||
| KNO3 | 56.6 g | ||
| (NH4)2SO4 | 9.26 g | ||
| MgSO4·7H2O | 3.7 g | ||
| KH2PO4 | 8 g | ||
| CaCL2·2H2O | 3.32 g | ||
| B5 micronutrient components (200×) | |||
| MnSO4·4H2O | 0.88 g | ||
| ZnSO4·7H2O | 0.3 g | ||
| H3BO3 | 0.32 g | ||
| Copper and cobalt components (2 000×) | |||
| CuSO4·5H2O | 0.05 g | ||
| CoCL2·6H2O | 0.05 g | ||
| KI | 1.7 g | ||
| Na2MoO4·2H2O | 0.5 g | ||
| Ferric salts components (200×) | |||
| FeSO4·7H2O | 5.57 g | ||
| Na-EDTA·2H2O | 7.45 g | ||
| NB organic components (200×) | |||
| Glycine | 0.4 g | ||
| Thiamine | 0.2 g | ||
| Pyridoxine | 0.1 g | ||
| Inositol | 20 g | ||
| Nicotinic acid | 0.1 g | ||
| D3 organic component (200×) | |||
| Glycine | 0.2 g | ||
| VB1 | 0.1 g | ||
| Thiamine | 0.1 g | ||
| Pyridoxine | 10 g | ||
| Nicotinic acid | 0.1 g | ||
| plant hormones and acetosyringone | |||
| National Aeronautic Association (NAA) | 0.2 g | ||
| 3-Indoleacetic acid (IAA) a | 0.2 g | ||
| Kinetin (KT) | 0.2 g | ||
| 6-Benzylaminopurine (6-BA) | 0.2 g | ||
| 2,4-Dichlorophenoxyacetic acid (2,4-D) | 0.2 g | ||
| Acetosyringone (AS) a | 200 mol | ||
| Induction medium | |||
| N6 macronutrient components (20×) | 50 mL | ||
| B5 micronutrient components (200×) | 5 mL | ||
| Copper and cobalt components (2 000×) | 0.5 mL | ||
| NB organic components (200×) | 5 mL | ||
| Ferric salts components (200×) | 5 mL | ||
| 2,4-D (0.2 mg/mL) | 12.5 mL | ||
| Casein hydrolysate | 0.3 g | ||
| Proline | 2.9 g | ||
| Sucrose | 30 g | ||
| PhytagelTM | 4 g | ||
| AAM medium | |||
| AAM macronutrient components (20×) | 50 mL | ||
| B5 micronutrient components (200×) | 5 mL | ||
| AAM organic components (100×) | 5 mL | ||
| Ferric salts components (200×) | 10 mL | ||
| AAM copper and cobalt components (2 000×) | 0.5 mL | ||
| Casein hydrolysate | 0.5 g | ||
| Glucose | 36 g | ||
| Sucrose | 68.5 g | ||
| L-Arginine | 0.18 g | ||
| L-Glutamine | 0.9 g | ||
| L-Asparagine | 0.3 g | ||
| AS (200 mmol/L) a | 0.5 mL | ||
| Co-culture medium | |||
| N6 macronutrient components (20×) | 50 mL | ||
| B5 micronutrient components (200×) | 5 mL | ||
| Copper and cobalt components (2 000×) | 0.5 mL | ||
| NB organic components (200×) | 5 mL | ||
| Ferric salt components (200×) | 5 mL | ||
| 2,4-D (0.2 mg/mL) | 12.5 mL | ||
| Casein hydrolysate | 0.3 g | ||
| Proline | 0.5 g | ||
| Glutamine | 0.5 g | ||
| Glucose | 10 g | ||
| Sucrose | 30 g | ||
| PhytagelTM | 4 g | ||
| AS (200 mmol/L) | 0.5 mL | ||
| Selection medium | |||
| MS macronutrient components (20×) | 50 mL | ||
| B5 micronutrient components (200×) | 50 mL | ||
| Copper and cobalt components (2 000×) | 5 mL | ||
| D3 organic components (100×) | 10 mL | ||
| Ferric salts components (200×) | 7.5 mL | ||
| 2,4-D (0.2 mg/mL) | 12.5 mL | ||
| Casein hydrolysate | 0.3 g | ||
| L-Glutamine | 0.5 g | ||
| Maltose | 30 g | ||
| PhytagelTM | 4 g | ||
| Hygromycin (50 mg/mL) | 0.6 mL | ||
| Carbenicillin (200 mg/mL) | 2 mL | ||
| Differentiation media | |||
| N6 macronutrient components (20×) | 50 mL | ||
| B5 micronutrient components (200×) | 50 mL | ||
| Copper and cobalt components (2 000×) | 5 mL | ||
| D3 organic components (100×) | 10 mL | ||
| Ferric salts components (200×) | 10 mL | ||
| Proline | 0.5 g | ||
| Casein hydrolysate | 0.8 g | ||
| L-Glutamine | 0.5 g | ||
| Maltose | 30 g | ||
| 6-BA (0.2 mg/mL) | 10 mL | ||
| KT (0.2 mg/mL) | 10 mL | ||
| NAA (0.2 mg/mL) | 1 mL | ||
| PhytagelTM | 4 g | ||
| IAA (0.2 mg/mL) | 1 mL | ||
| Rooting media | |||
| MS macronutrient components (20×) | 50 mL | ||
| MS micronutrient components (1,000×) | 1 mL | ||
| Copper and cobalt components (2 000×) | 0.5 mL | ||
| MS organic components (200×) | 5 mL | ||
| Ferric salts components (200×) | 5 mL | ||
| Sucrose | 30 g | ||
| Casein hydrolysate | 1 g | ||
| NAA (0.2 mg/mL) | 2.5 mL | ||
| PhytagelTM | 4 g | ||
| IAA (0.2 mg/mL) | 2.5 mL | ||
| [1] | Lin Y J, Zhang Q F. 2005. Optimising the tissue culture conditions for high efficiency transformation of indica rice. Plant Cell Rep, 23(8): 540-547. |
| [2] | Wang F, Tian B. 2001. Neurospora NADP-glutamate dehydrogenases and its expression in E. coli and transgenic plant. Chin Sci Bull, 46(2): 1029-1032. |
| [3] | Yan L, Gong Y Y, Luo Q, Dai G X, Teng Z N, He Y, Wu X X, Liu C, Tang D Y, Ye N H, Deng G F, Lin J Z, Liu X M. 2020. Heterologous expression of fungal AcGDH alleviates ammonium toxicity and suppresses photorespiration, thereby improving drought tolerance in rice. Plant Sci, 305(2): 110769. |
| [4] | Yuan L P. 1997. Hybrid rice breeding for super high yield. Hybrid Rice, 12: 1-6. (in Chinese) |
| No related articles found! |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||