
Rice Science ›› 2023, Vol. 30 ›› Issue (6): 598-612.DOI: 10.1016/j.rsci.2023.06.003
• Research Papers • Previous Articles Next Articles
Ji Dongling1, Xiao Wenhui1, Sun Zhiwei1, Liu Lijun1, Gu Junfei1, Zhang Hao1, Matthew Tom Harrison3, Liu Ke3, Wang Zhiqin1, Wang Weilu1,2( )
)
Received:2023-03-08
Accepted:2023-06-30
Online:2023-11-28
Published:2023-08-10
Contact:
WANG Weilu (weiluwang868@yzu.edu.cn)
Ji Dongling, Xiao Wenhui, Sun Zhiwei, Liu Lijun, Gu Junfei, Zhang Hao, Matthew Tom Harrison, Liu Ke, Wang Zhiqin, Wang Weilu. Translocation and Distribution of Carbon-Nitrogen in Relation to Rice Yield and Grain Quality as Affected by High Temperature at Early Panicle Initiation Stage[J]. Rice Science, 2023, 30(6): 598-612.
Add to citation manager EndNote|Ris|BibTeX
| Cultivar | Treatment | Grain yield (g/pot) | Panicle number per pot | Spikelet number per panicle | Grain-filling rate (%) | 1000-grain weight (g) | Harvest index (%) |
|---|---|---|---|---|---|---|---|
| Yangdao 6 | NT | 71.4 ± 3.7 a | 18.6 ± 1.2 a | 164.5 ± 2.6 a | 87.0 ± 0.4 a | 26.8 ± 0.4 a | 54.60 ± 0.03 a |
| HTS | 50.9 ± 2.4 b | 18.2 ± 1.7 a | 139.7 ± 6.7 b | 76.6 ± 0.6 b | 26.1 ± 0.1 b | 47.00 ± 0.05 bc | |
| Jinxiangyu 1 | NT | 50.6 ± 1.8 b | 17.2 ± 1.2 a | 134.8 ± 4.8 b | 90.5 ± 0.3 a | 24.1 ± 0.4 c | 50.20 ± 0.02 b |
| HTS | 38.2 ± 3.0 c | 17.0 ± 0.9 a | 125.7 ± 7.3 c | 77.1 ± 0.6 b | 23.2 ± 0.2 d | 43.90 ± 0.03 c | |
| Analysis of variance | |||||||
| Cultivar (C) | ** | ns | ** | ns | ** | * | |
| Treatment (T) | ** | ns | ** | ** | ** | ** | |
| C × T | ns | ns | * | ns | ns | ns | |
Table 1. Effects of high temperature stress at early panicle initiation stage on grain yield and its components.
| Cultivar | Treatment | Grain yield (g/pot) | Panicle number per pot | Spikelet number per panicle | Grain-filling rate (%) | 1000-grain weight (g) | Harvest index (%) |
|---|---|---|---|---|---|---|---|
| Yangdao 6 | NT | 71.4 ± 3.7 a | 18.6 ± 1.2 a | 164.5 ± 2.6 a | 87.0 ± 0.4 a | 26.8 ± 0.4 a | 54.60 ± 0.03 a |
| HTS | 50.9 ± 2.4 b | 18.2 ± 1.7 a | 139.7 ± 6.7 b | 76.6 ± 0.6 b | 26.1 ± 0.1 b | 47.00 ± 0.05 bc | |
| Jinxiangyu 1 | NT | 50.6 ± 1.8 b | 17.2 ± 1.2 a | 134.8 ± 4.8 b | 90.5 ± 0.3 a | 24.1 ± 0.4 c | 50.20 ± 0.02 b |
| HTS | 38.2 ± 3.0 c | 17.0 ± 0.9 a | 125.7 ± 7.3 c | 77.1 ± 0.6 b | 23.2 ± 0.2 d | 43.90 ± 0.03 c | |
| Analysis of variance | |||||||
| Cultivar (C) | ** | ns | ** | ns | ** | * | |
| Treatment (T) | ** | ns | ** | ** | ** | ** | |
| C × T | ns | ns | * | ns | ns | ns | |
| Cultivar | Treatment | Grain length (mm) | Grain width (mm) | Length-width ratio |
|---|---|---|---|---|
| Yangdao 6 | NT | 9.33 ± 0.09 a | 2.78 ± 0.03 b | 3.36 ± 0.05 a |
| HTS | 8.78 ± 0.07 b | 2.70 ± 0.05 b | 3.25 ± 0.06 b | |
| Jinxiangyu 1 | NT | 6.97 ± 0.06 c | 3.52 ± 0.06 a | 1.98 ± 0.04 c |
| HTS | 6.64 ± 0.05 d | 3.47 ± 0.15 a | 1.92 ± 0.08 c | |
| Analysis of variance | ||||
| Cultivar (C) | ** | ** | ** | |
| Treatment (T) | ** | ns | ** | |
| C × T | ** | ns | ns | |
Table 2. Effects of high temperature stress at early panicle initiation stage on grain morphology.
| Cultivar | Treatment | Grain length (mm) | Grain width (mm) | Length-width ratio |
|---|---|---|---|---|
| Yangdao 6 | NT | 9.33 ± 0.09 a | 2.78 ± 0.03 b | 3.36 ± 0.05 a |
| HTS | 8.78 ± 0.07 b | 2.70 ± 0.05 b | 3.25 ± 0.06 b | |
| Jinxiangyu 1 | NT | 6.97 ± 0.06 c | 3.52 ± 0.06 a | 1.98 ± 0.04 c |
| HTS | 6.64 ± 0.05 d | 3.47 ± 0.15 a | 1.92 ± 0.08 c | |
| Analysis of variance | ||||
| Cultivar (C) | ** | ** | ** | |
| Treatment (T) | ** | ns | ** | |
| C × T | ** | ns | ns | |
| Cultivar | Treatment | No. of primary branches per panicle | No. of secondary branches per panicle | Panicle length (cm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Differentiated | Degenerated | Surviving | Differentiated | Degenerated | Surviving | |||||||
| Yangdao 6 | NT | 11.0 ± 0.1 b | 0.0 ± 0.1 c | 11.0 ± 0.1 b | 47.5 ± 1.8 a | 13.8 ± 0.9 b | 33.7 ± 1.7 a | 23.4 ± 1.0 a | ||||
| HTS | 11.5 ± 0.2 b | 0.1 ± 0.1 bc | 11.4 ± 0.1 b | 44.2 ± 2.0 a | 15.3 ± 0.7 b | 29.0 ± 2.6 b | 21.4 ± 0.7 b | |||||
| Jinxiangyu 1 | NT | 12.8 ± 0.1 a | 0.3 ± 0.0 b | 12.5 ± 0.1 a | 39.4 ± 0.4 b | 17.5 ± 0.3 a | 21.3 ± 0.5 c | 16.1 ± 0.2 c | ||||
| HTS | 13.1 ± 0.2 a | 0.7 ± 0.2 a | 12.4 ± 0.4 a | 36.0 ± 0.4 b | 18.1 ± 0.4 a | 18.5 ± 0.7 c | 14.4 ± 0.1 d | |||||
| Analysis of variance | ||||||||||||
| Cultivar (C) | ** | ** | ** | ** | ** | ** | ** | |||||
| Treatment (T) | * | * | ns | * | * | * | ** | |||||
| C × T | ns | ns | ns | ns | ns | ns | ns | |||||
Table 3. Effects of high temperature stress at early panicle initiation stage on differentiation and degeneration of branches and panicle length.
| Cultivar | Treatment | No. of primary branches per panicle | No. of secondary branches per panicle | Panicle length (cm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Differentiated | Degenerated | Surviving | Differentiated | Degenerated | Surviving | |||||||
| Yangdao 6 | NT | 11.0 ± 0.1 b | 0.0 ± 0.1 c | 11.0 ± 0.1 b | 47.5 ± 1.8 a | 13.8 ± 0.9 b | 33.7 ± 1.7 a | 23.4 ± 1.0 a | ||||
| HTS | 11.5 ± 0.2 b | 0.1 ± 0.1 bc | 11.4 ± 0.1 b | 44.2 ± 2.0 a | 15.3 ± 0.7 b | 29.0 ± 2.6 b | 21.4 ± 0.7 b | |||||
| Jinxiangyu 1 | NT | 12.8 ± 0.1 a | 0.3 ± 0.0 b | 12.5 ± 0.1 a | 39.4 ± 0.4 b | 17.5 ± 0.3 a | 21.3 ± 0.5 c | 16.1 ± 0.2 c | ||||
| HTS | 13.1 ± 0.2 a | 0.7 ± 0.2 a | 12.4 ± 0.4 a | 36.0 ± 0.4 b | 18.1 ± 0.4 a | 18.5 ± 0.7 c | 14.4 ± 0.1 d | |||||
| Analysis of variance | ||||||||||||
| Cultivar (C) | ** | ** | ** | ** | ** | ** | ** | |||||
| Treatment (T) | * | * | ns | * | * | * | ** | |||||
| C × T | ns | ns | ns | ns | ns | ns | ns | |||||
| Cultivar | Treatment | Total number of differentiated spikelets per panicle | No. of spikelets on primary branch per panicle | No. of spikelets on secondary branch per panicle | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Differentiated | Degenerated | Surviving | Differentiated | Degenerated | Surviving | |||||
| Yangdao 6 | NT | 168.2 ± 6.2 a | 62.2 ± 0.8 b | 0.1 ± 0.0 a | 62.2 ± 0.8 b | 106.0 ± 5.3 a | 2.6 ± 0.7 d | 103.4 ± 4.6 a | ||
| HTS | 139.4 ± 1.5 b | 61.4 ± 0.7 b | 0.3 ± 0.1 a | 62.0 ± 0.9 b | 82.9 ± 3.9 b | 8.5 ± 0.8 b | 74.4 ± 3.2 b | |||
| Jinxiangyu 1 | NT | 139.6 ± 0.6 b | 76.6 ± 1.1 a | 1.2 ± 1.0 a | 75.4 ± 0.4 a | 63.0 ± 1.5 c | 5.9 ± 0.7 c | 55.7 ± 2.1 c | ||
| HTS | 127.3 ± 1.1 c | 75.3 ± 1.6 a | 0.9 ± 0.5 a | 74.4 ± 1.6 a | 52.0 ± 1.2 d | 10.7 ± 1.1 a | 41.3 ± 2.0 d | |||
| Analysis of variance | ||||||||||
| Cultivar (C) | ** | ** | * | ** | ** | ** | ** | |||
| Treatment (T) | ** | ns | ns | ns | ** | ** | ** | |||
| C × T | * | ns | ns | ns | ns | ns | * | |||
Table 4. Effects of high temperature stress at early panicle initiation stage on differentiation and degeneration of spikelets.
| Cultivar | Treatment | Total number of differentiated spikelets per panicle | No. of spikelets on primary branch per panicle | No. of spikelets on secondary branch per panicle | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Differentiated | Degenerated | Surviving | Differentiated | Degenerated | Surviving | |||||
| Yangdao 6 | NT | 168.2 ± 6.2 a | 62.2 ± 0.8 b | 0.1 ± 0.0 a | 62.2 ± 0.8 b | 106.0 ± 5.3 a | 2.6 ± 0.7 d | 103.4 ± 4.6 a | ||
| HTS | 139.4 ± 1.5 b | 61.4 ± 0.7 b | 0.3 ± 0.1 a | 62.0 ± 0.9 b | 82.9 ± 3.9 b | 8.5 ± 0.8 b | 74.4 ± 3.2 b | |||
| Jinxiangyu 1 | NT | 139.6 ± 0.6 b | 76.6 ± 1.1 a | 1.2 ± 1.0 a | 75.4 ± 0.4 a | 63.0 ± 1.5 c | 5.9 ± 0.7 c | 55.7 ± 2.1 c | ||
| HTS | 127.3 ± 1.1 c | 75.3 ± 1.6 a | 0.9 ± 0.5 a | 74.4 ± 1.6 a | 52.0 ± 1.2 d | 10.7 ± 1.1 a | 41.3 ± 2.0 d | |||
| Analysis of variance | ||||||||||
| Cultivar (C) | ** | ** | * | ** | ** | ** | ** | |||
| Treatment (T) | ** | ns | ns | ns | ** | ** | ** | |||
| C × T | * | ns | ns | ns | ns | ns | * | |||
| Cultivar | Treatment | Brown rice rate (%) | Milled rice rate (%) | Head rice rate (%) | Gel consistency (mm) | Protein content (%) | Amylose content (%) |
|---|---|---|---|---|---|---|---|
| Yangdao 6 | NT | 81.70 ± 3.13 a | 76.30 ± 1.03 a | 67.80 ± 3.23 a | 68.70 ± 3.84 b | 8.55 ± 0.56 bc | 16.70 ± 0.34 b |
| HTS | 81.20 ± 2.77 a | 70.70 ± 3.85 bc | 43.50 ± 4.85 c | 41.20 ± 1.86 d | 10.10 ± 1.03 a | 20.30 ± 0.75 a | |
| Jinxiangyu 1 | NT | 82.70 ± 1.51 a | 73.30 ± 3.18 b | 69.70 ± 2.85 a | 73.80 ± 13.01 a | 8.22 ± 0.29 c | 13.50 ± 0.96 c |
| HTS | 81.70 ± 3.08 a | 68.40 ± 2.54 c | 50.60 ± 3.69 b | 59.20 ± 2.95 c | 9.11 ± 0.89 b | 16.10 ± 1.73 d | |
| Analysis of variance | |||||||
| Cultivar (C) | ns | * | * | ** | * | ** | |
| Treatment (T) | ns | ** | ** | ** | ** | ** | |
| C × T | ns | ns | ns | ns | ns | ns | |
Table 5. Effects of high temperature stress at early panicle initiation stage on milling, cooking, eating, and nutrition qualities.
| Cultivar | Treatment | Brown rice rate (%) | Milled rice rate (%) | Head rice rate (%) | Gel consistency (mm) | Protein content (%) | Amylose content (%) |
|---|---|---|---|---|---|---|---|
| Yangdao 6 | NT | 81.70 ± 3.13 a | 76.30 ± 1.03 a | 67.80 ± 3.23 a | 68.70 ± 3.84 b | 8.55 ± 0.56 bc | 16.70 ± 0.34 b |
| HTS | 81.20 ± 2.77 a | 70.70 ± 3.85 bc | 43.50 ± 4.85 c | 41.20 ± 1.86 d | 10.10 ± 1.03 a | 20.30 ± 0.75 a | |
| Jinxiangyu 1 | NT | 82.70 ± 1.51 a | 73.30 ± 3.18 b | 69.70 ± 2.85 a | 73.80 ± 13.01 a | 8.22 ± 0.29 c | 13.50 ± 0.96 c |
| HTS | 81.70 ± 3.08 a | 68.40 ± 2.54 c | 50.60 ± 3.69 b | 59.20 ± 2.95 c | 9.11 ± 0.89 b | 16.10 ± 1.73 d | |
| Analysis of variance | |||||||
| Cultivar (C) | ns | * | * | ** | * | ** | |
| Treatment (T) | ns | ** | ** | ** | ** | ** | |
| C × T | ns | ns | ns | ns | ns | ns | |
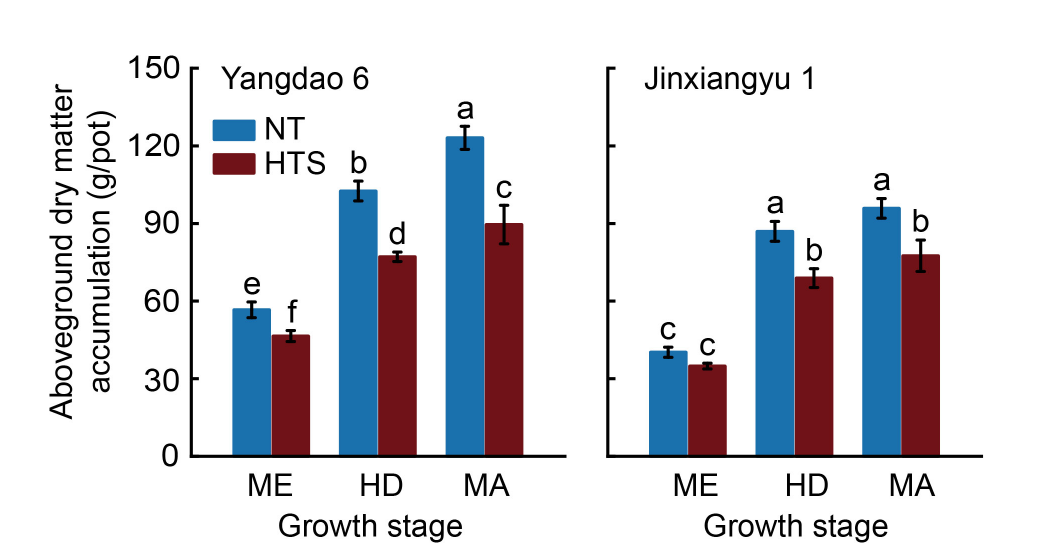
Fig. 1. Effects of high temperature stress at early panicle initiation stage on aboveground dry matter accumulation at meiosis (ME), heading (HD), and maturity (MA) stages. NT and HTS represent normal temperature and high temperature stress treatments, respectively. Data are Mean ± SD (n = 3). Different lowercase letters above bars indicate statistical significances at the P ≤ 0.05 level within the same measurement time.
| Cultivar | Treatment | Root | Stem | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Meiosis stage | Heading stage | Maturity stage | Meiosis stage | Heading stage | Maturity stage | ||||
| Dry matter distribution rate | |||||||||
| Yangdao 6 | NT | 20.2 ± 1.4 a | 16.7 ± 0.5 b | 11.9 ± 0.3 b | 51.3 ± 0.4 c | 44.9 ± 2.5 ab | 27.8 ± 0.6 ab | ||
| HTS | 21.6 ± 0.7 a | 19.2 ± 0.6 a | 14.9 ± 1.0 a | 48.8 ± 0.7 d | 39.5 ± 1.4 c | 26.3 ± 1.6 b | |||
| Jinxiangyu 1 | NT | 22.2 ± 0.5 a | 18.0 ± 1.5 ab | 14.0 ± 0.3 ab | 53.1 ± 0.5 b | 45.2 ± 2.8 a | 29.9 ± 1.8 a | ||
| HTS | 20.9 ± 0.9 a | 18.1 ± 0.3 ab | 15.1 ± 1.3 a | 54.3 ± 0.4 a | 43.1 ± 1.9 b | 31.0 ± 1.5 a | |||
| Analysis of variance | |||||||||
| Cultivar (C) | ns | ns | ns | ** | ** | * | |||
| Treatment (T) | ns | ns | * | ns | ** | ns | |||
| C × T | ns | ns | ns | ** | * | ns | |||
| Leaf | Panicle | ||||||||
| Meiosis stage | Heading stage | Maturity stage | Heading stage | Maturity stage | |||||
| Yangdao 6 | NT | 28.5 ± 1.0 a | 23.6 ± 1.8 a | 11.0 ± 0.4 c | 14.8 ± 1.0 b | 49.3 ± 1.1 a | |||
| HTS | 29.6 ± 0.5 a | 24.2 ± 1.9 a | 14.0 ± 1.0 ab | 17.1 ± 0.1 a | 44.7 ± 2.4 b | ||||
| Jinxiangyu 1 | NT | 24.7 ± 0.9 b | 23.2 ± 1.0 a | 12.4 ± 0.6 bc | 13.5 ± 0.2 b | 43.7 ± 1.9 b | |||
| HTS | 24.8 ± 0.7 b | 24.4 ± 1.5 a | 14.5 ± 1.7 a | 14.5 ± 1.0 b | 39.4 ± 3.1 c | ||||
| Analysis of variance | |||||||||
| Cultivar (C) | ** | ns | ns | ** | ** | ||||
| Treatment (T) | ns | ns | ** | * | ** | ||||
| C × T | ns | ns | ns | ns | ns | ||||
| Nitrogen distribution rate | |||||||||
| Root | Stem | ||||||||
| Meiosis stage | Heading stage | Maturity stage | Meiosis stage | Heading stage | Maturity stage | ||||
| Yangdao 6 | NT | 11.5 ± 1.0 b | 9.3 ± 0.7 a | 7.0 ± 0.9 b | 41.3 ± 1.4 a | 33.3 ± 3.4 a | 16.1 ± 1.5 a | ||
| HTS | 13.3 ± 0.4 b | 10.4 ± 0.7 a | 9.0 ± 0.5 a | 36.0 ± 0.2 b | 26.7 ± 2.8 b | 15.7 ± 1.0 a | |||
| Jinxiangyu 1 | NT | 16.8 ± 1.0 a | 10.6 ± 1.1 a | 8.5 ± 0.6 ab | 38.4 ± 1.6 ab | 32.9 ± 3.6 a | 11.0 ± 0.6 b | ||
| HTS | 17.6 ± 1.2 a | 10.2 ± 0.7 a | 8.5 ± 0.5 ab | 36.9 ± 1.4 b | 29.0 ± 2.1 b | 12.1 ± 0.6 b | |||
| Analysis of variance | |||||||||
| Cultivar (C) | ** | ns | ns | ns | ns | ** | |||
| Treatment (T) | ns | ns | ns | * | ** | ns | |||
| C × T | ns | ns | ns | ns | ns | ns | |||
| Leaf | Panicle | ||||||||
| Meiosis stage | Heading stage | Maturity stage | Heading stage | Maturity stage | |||||
| Yangdao 6 | NT | 47.3 ± 1.7 ab | 43.8 ± 3.3 a | 9.6 ± 0.7 bc | 13.6 ± 0.8 b | 67.3 ± 3.6 b | |||
| HTS | 50.7 ± 0.3 a | 45.4 ± 2.1 a | 12.9 ± 0.6 a | 17.5 ± 1.5 a | 62.5 ± 2.2 c | ||||
| Jinxiangyu 1 | NT | 44.8 ± 1.7 b | 43.4 ± 1.1 a | 8.6 ± 0.6 c | 13.0 ± 0.8 b | 72.0 ± 5.5 a | |||
| HTS | 45.5 ± 2.7 b | 46.8 ± 2.2 a | 11.1 ± 0.9 b | 14.0 ± 0.7 b | 68.4 ± 3.3 b | ||||
| Analysis of variance | |||||||||
| Cultivar (C) | * | ns | * | * | ** | ||||
| Treatment (T) | ns | ns | ** | * | ** | ||||
| C × T | ns | ns | ns | ns | ns | ||||
Table 6. Effects of high temperature stress at early panicle initiation stage on dry matter distribution rate and nitrogen distribution rate at meiosis, heading, and maturity stages.
| Cultivar | Treatment | Root | Stem | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Meiosis stage | Heading stage | Maturity stage | Meiosis stage | Heading stage | Maturity stage | ||||
| Dry matter distribution rate | |||||||||
| Yangdao 6 | NT | 20.2 ± 1.4 a | 16.7 ± 0.5 b | 11.9 ± 0.3 b | 51.3 ± 0.4 c | 44.9 ± 2.5 ab | 27.8 ± 0.6 ab | ||
| HTS | 21.6 ± 0.7 a | 19.2 ± 0.6 a | 14.9 ± 1.0 a | 48.8 ± 0.7 d | 39.5 ± 1.4 c | 26.3 ± 1.6 b | |||
| Jinxiangyu 1 | NT | 22.2 ± 0.5 a | 18.0 ± 1.5 ab | 14.0 ± 0.3 ab | 53.1 ± 0.5 b | 45.2 ± 2.8 a | 29.9 ± 1.8 a | ||
| HTS | 20.9 ± 0.9 a | 18.1 ± 0.3 ab | 15.1 ± 1.3 a | 54.3 ± 0.4 a | 43.1 ± 1.9 b | 31.0 ± 1.5 a | |||
| Analysis of variance | |||||||||
| Cultivar (C) | ns | ns | ns | ** | ** | * | |||
| Treatment (T) | ns | ns | * | ns | ** | ns | |||
| C × T | ns | ns | ns | ** | * | ns | |||
| Leaf | Panicle | ||||||||
| Meiosis stage | Heading stage | Maturity stage | Heading stage | Maturity stage | |||||
| Yangdao 6 | NT | 28.5 ± 1.0 a | 23.6 ± 1.8 a | 11.0 ± 0.4 c | 14.8 ± 1.0 b | 49.3 ± 1.1 a | |||
| HTS | 29.6 ± 0.5 a | 24.2 ± 1.9 a | 14.0 ± 1.0 ab | 17.1 ± 0.1 a | 44.7 ± 2.4 b | ||||
| Jinxiangyu 1 | NT | 24.7 ± 0.9 b | 23.2 ± 1.0 a | 12.4 ± 0.6 bc | 13.5 ± 0.2 b | 43.7 ± 1.9 b | |||
| HTS | 24.8 ± 0.7 b | 24.4 ± 1.5 a | 14.5 ± 1.7 a | 14.5 ± 1.0 b | 39.4 ± 3.1 c | ||||
| Analysis of variance | |||||||||
| Cultivar (C) | ** | ns | ns | ** | ** | ||||
| Treatment (T) | ns | ns | ** | * | ** | ||||
| C × T | ns | ns | ns | ns | ns | ||||
| Nitrogen distribution rate | |||||||||
| Root | Stem | ||||||||
| Meiosis stage | Heading stage | Maturity stage | Meiosis stage | Heading stage | Maturity stage | ||||
| Yangdao 6 | NT | 11.5 ± 1.0 b | 9.3 ± 0.7 a | 7.0 ± 0.9 b | 41.3 ± 1.4 a | 33.3 ± 3.4 a | 16.1 ± 1.5 a | ||
| HTS | 13.3 ± 0.4 b | 10.4 ± 0.7 a | 9.0 ± 0.5 a | 36.0 ± 0.2 b | 26.7 ± 2.8 b | 15.7 ± 1.0 a | |||
| Jinxiangyu 1 | NT | 16.8 ± 1.0 a | 10.6 ± 1.1 a | 8.5 ± 0.6 ab | 38.4 ± 1.6 ab | 32.9 ± 3.6 a | 11.0 ± 0.6 b | ||
| HTS | 17.6 ± 1.2 a | 10.2 ± 0.7 a | 8.5 ± 0.5 ab | 36.9 ± 1.4 b | 29.0 ± 2.1 b | 12.1 ± 0.6 b | |||
| Analysis of variance | |||||||||
| Cultivar (C) | ** | ns | ns | ns | ns | ** | |||
| Treatment (T) | ns | ns | ns | * | ** | ns | |||
| C × T | ns | ns | ns | ns | ns | ns | |||
| Leaf | Panicle | ||||||||
| Meiosis stage | Heading stage | Maturity stage | Heading stage | Maturity stage | |||||
| Yangdao 6 | NT | 47.3 ± 1.7 ab | 43.8 ± 3.3 a | 9.6 ± 0.7 bc | 13.6 ± 0.8 b | 67.3 ± 3.6 b | |||
| HTS | 50.7 ± 0.3 a | 45.4 ± 2.1 a | 12.9 ± 0.6 a | 17.5 ± 1.5 a | 62.5 ± 2.2 c | ||||
| Jinxiangyu 1 | NT | 44.8 ± 1.7 b | 43.4 ± 1.1 a | 8.6 ± 0.6 c | 13.0 ± 0.8 b | 72.0 ± 5.5 a | |||
| HTS | 45.5 ± 2.7 b | 46.8 ± 2.2 a | 11.1 ± 0.9 b | 14.0 ± 0.7 b | 68.4 ± 3.3 b | ||||
| Analysis of variance | |||||||||
| Cultivar (C) | * | ns | * | * | ** | ||||
| Treatment (T) | ns | ns | ** | * | ** | ||||
| C × T | ns | ns | ns | ns | ns | ||||

Fig. 2. Effects of high temperature stress at early panicle initiation stage on nitrogen accumulation at meiosis (ME), heading (HD), and maturity (MA) stages. NT and HTS represent normal temperature and high temperature stress treatments, respectively. Data are Mean ± SD (n = 3). Different lowercase letters above bars indicate statistical significances at the P ≤ 0.05 level within the same measurement time.
| Cultivar | Treatment | Dry matter | Nitrogen | |||||
|---|---|---|---|---|---|---|---|---|
| Translocation amount (g) | Translocation rate (%) | Contribution rate (%) | Translocation amount (mg) | Translocation rate (%) | Contribution rate (%) | |||
| Yangdao 6 | NT | 30.0 ± 3.7 a | 53.5 ± 3.5 ab | 45.4 ± 2.8 b | 877.2 ± 79.0 a | 64.6 ± 4.2 b | 70.0 ± 5.2 a | |
| HTS | 18.4 ± 1.4 b | 39.7 ± 1.7 c | 39.0 ± 1.5 c | 437.0 ± 29.8 b | 49.1 ± 2.4 c | 43.9 ± 1.8 c | ||
| Jinxiangyu 1 | NT | 25.4 ± 1.7 a | 62.7 ± 6.0 a | 52.6 ± 3.9 a | 971.8 ± 91.1 a | 74.0 ± 3.0 a | 77.5 ± 1.9 a | |
| HTS | 15.3 ± 1.1 b | 43.6 ± 2.0 bc | 43.3 ± 2.2 bc | 590.3 ± 39.5 b | 61.7 ± 2.0 b | 54.8 ± 5.1 b | ||
| Analysis of variance | ||||||||
| Cultivar (C) | ns | ns | * | * | ** | ** | ||
| Treatment (T) | ** | ** | ** | ** | ** | ** | ||
| C × T | ns | ns | ns | ns | ns | ns | ||
Table 7. Effects of high temperature stress at early panicle initiation stage on pre-anthesis aboveground dry matter and nitrogen translocation amounts, and their translocation efficiencies, and contribution rates of translocation amount to grain yield.
| Cultivar | Treatment | Dry matter | Nitrogen | |||||
|---|---|---|---|---|---|---|---|---|
| Translocation amount (g) | Translocation rate (%) | Contribution rate (%) | Translocation amount (mg) | Translocation rate (%) | Contribution rate (%) | |||
| Yangdao 6 | NT | 30.0 ± 3.7 a | 53.5 ± 3.5 ab | 45.4 ± 2.8 b | 877.2 ± 79.0 a | 64.6 ± 4.2 b | 70.0 ± 5.2 a | |
| HTS | 18.4 ± 1.4 b | 39.7 ± 1.7 c | 39.0 ± 1.5 c | 437.0 ± 29.8 b | 49.1 ± 2.4 c | 43.9 ± 1.8 c | ||
| Jinxiangyu 1 | NT | 25.4 ± 1.7 a | 62.7 ± 6.0 a | 52.6 ± 3.9 a | 971.8 ± 91.1 a | 74.0 ± 3.0 a | 77.5 ± 1.9 a | |
| HTS | 15.3 ± 1.1 b | 43.6 ± 2.0 bc | 43.3 ± 2.2 bc | 590.3 ± 39.5 b | 61.7 ± 2.0 b | 54.8 ± 5.1 b | ||
| Analysis of variance | ||||||||
| Cultivar (C) | ns | ns | * | * | ** | ** | ||
| Treatment (T) | ** | ** | ** | ** | ** | ** | ||
| C × T | ns | ns | ns | ns | ns | ns | ||
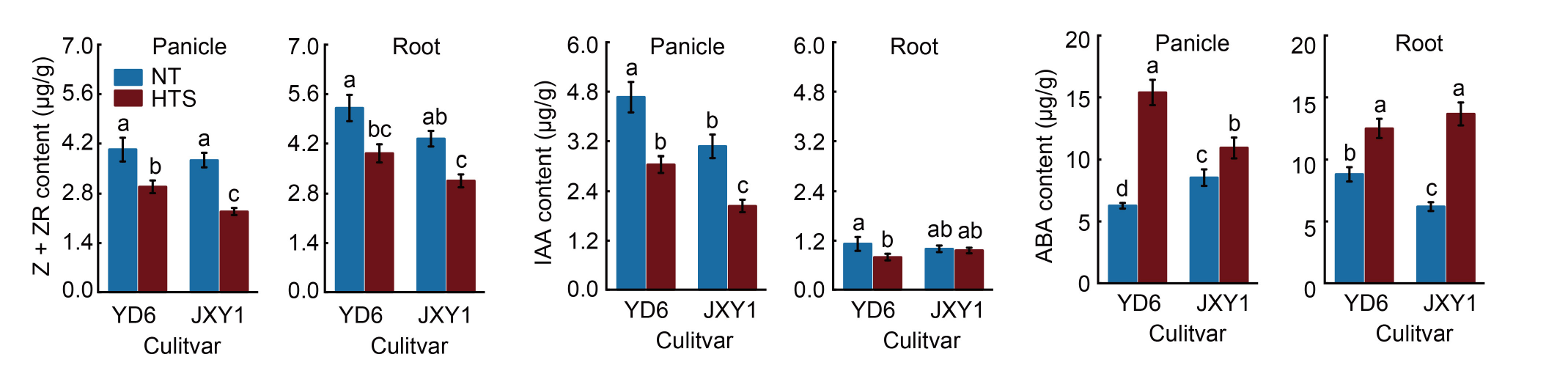
Fig. 3. Effects of high temperature stress at early panicle initiation stage on plant hormones. NT and HTS represent normal temperature and high temperature stress treatments, respectively. Z + ZR, IAA, and ABA represent zeatin + zeatin riboside, auxin, and abscisic acid, respectively. YD6 and JXY1 represent Yangdao 6 and Jinxiangyu 1, respectively. Data are Mean ± SD (n = 3). Different lowercase letters above bars indicate statistical significances at the P ≤ 0.05 level within the same measurement time.
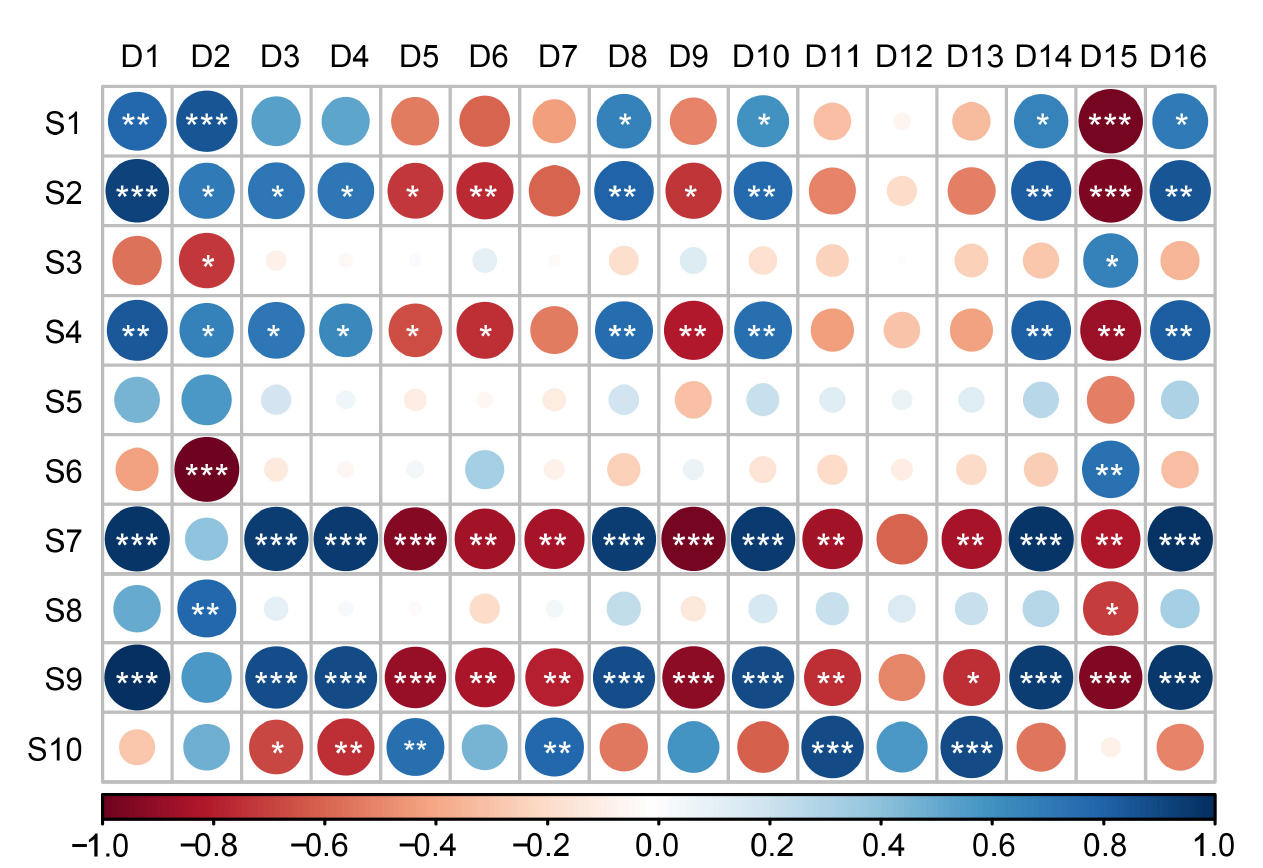
Fig. 4. Correlation analysis of hormone, dry matter and nitrogen accumulation and translocation with yield and spikelet traits. D1, The number of spikelets per panicle; D2, Grain-filling rate; D3, Panicle length; D4, Grain length; D5, The number of differentiated primary branches per panicle; D6, The number of degenerated primary branches per panicle; D7, The number of surviving primary branches per panicle; D8, The number of differentiated secondary branches per panicle; D9, The number of degenerated secondary branches per panicle; D10, The number of surviving secondary branches per panicle; D11, The number of differentiated spikelets on primary branches per panicle; D12, The number of degenerated spikelets on primary branch per panicle; D13, The number of surviving spikelets on primary branch per panicle; D14, The number of differentiated spikelets on secondary branch per panicle; D15, The number of degenerated spikelets on secondary branch per panicle; D16, The number of surviving spikelets on secondary branch per panicle; S1, zeatin + zeatin riboside (Z + ZR) content in panicles; S2, Auxin (IAA) content in panicles; S3, Abscisic acid (ABA) content in panicles; S4, Z + ZR content in roots; S5, IAA content in roots; S6, ABA content in roots; S7, Aboveground dry matter accumulation at the meiosis stage; S8, Aboveground dry matter accumulation at the meiosis to heading stage; S9, Aboveground nitrogen accumulation at the meiosis stage; S10, Aboveground dry matter accumulation at the meiosis to heading stage. Red and blue circles indicate negative and positive correlations between parameters, respectively. The darker the color, the higher the correlation. *, **, and *** indicate significant differences at P ≤ 0.05, P ≤ 0.01, and P ≤ 0.001, respectively.
| [1] | Cai C, Yin X Y, He S Q, Jiang W Y, Si C F, Struik P C, Luo W H, Li G, Xie Y T, Xiong Y, Pan G X. 2016. Responses of wheat and rice to factorial combinations of ambient and elevated CO2 and temperature in FACE experiments. Glob Change Biol, 22(2): 856-874. |
| [2] | Chen C, Huang J L, Zhu L Y, Shah F, Nie L X, Cui K H, Peng S B. 2013. Varietal difference in the response of rice chalkiness to temperature during ripening phase across different sowing dates. Field Crops Res, 151: 85-91. |
| [3] | Chen C P, Sakai H, Tokida T, Usui Y, Nakamura H, Hasegawa T. 2014. Do the rich always become richer? Characterizing the leaf physiological response of the high-yielding rice cultivar Takanari to free-air CO2 enrichment. Plant Cell Physiol, 55(2): 381-391. |
| [4] | Chen Y H, Chen H Z, Xiang J, Zhang Y K, Wang Z G, Zhu D F, Wang J K, Zhang Y P, Wang Y L. 2021. Rice spikelet formation inhibition caused by decreased sugar utilization under high temperature is associated with brassinolide decomposition. Environ Exp Bot, 190: 104585. |
| [5] | Fitzgerald M A, Resurreccion A P. 2009. Maintaining the yield of edible rice in a warming world. Funct Plant Biol, 36(12): 1037-1045. |
| [6] | Gaupp F, Hall J, Hochrainer-Stigler S, Dadson S. 2020. Changing risks of simultaneous global breadbasket failure. Nat Clim Chang, 10(1): 54-57. |
| [7] | Gourdji S M, Sibley A M, Lobell D B. 2013. Global crop exposure to critical high temperatures in the reproductive period: Historical trends and future projections. Environ Res Lett, 8(2): 024041. |
| [8] | Guo X H, Huang B W, Zhang H, Cai C, Li G, Li H Z, Zhang Y L, Struik P C, Liu Z J, Dong M M, Ni R B, Pan G X, Liu X Y, Chen W P, Luo W H, Yin X Y. 2022. T-FACE studies reveal that increased temperature exerts an effect opposite to that of elevated CO2 on nutrient concentration and bioavailability in rice and wheat grains. Food Energy Secur, 11(1): e336. |
| [9] | Hu Q Q, Wang W C, Lu Q F, Huang J L, Peng S B, Cui K H. 2021. Abnormal anther development leads to lower spikelet fertility in rice (Oryza sativa L.) under high temperature during the panicle initiation stage. BMC Plant Biol, 21(1): 1-17. |
| [10] | Hu Z J, Lu S J, Wang M J, He H H, Sun L, Wang H R, Liu X H, Jiang L, Sun J L, Xin X Y, Kong W, Chu C C, Xue H W, Yang J S, Luo X J, Liu J X. 2018. A novel QTL qTGW3 encodes the GSK3/SHAGGY-like kinase OsGSK5/OsSK41 that interacts with OsARF4 to negatively regulate grain size and weight in rice. Mol Plant, 11(5): 736-749. |
| [11] | Huang R Y, Jiang L R, Zheng J S, Wang T S, Wang H C, Huang Y M, Hong Z L. 2013. Genetic bases of rice grain shape: So many genes, so little known. Trends Plant Sci, 18(4): 218-226. |
| [12] | IPCC. 2022. Climate Change 2022:Impacts, Adaptation, and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK and New York, USA: Cambridge University Press: 3056. |
| [13] | Jagadish S V K, Craufurd P Q, Wheeler T R. 2007. High temperature stress and spikelet fertility in rice (Oryza sativa L.). J Exp Bot, 58(7): 1627-1635. |
| [14] | Kim H Y, Lim S S, Kwak J H, Lee D S, Lee S M, Ro H M, Choi W J. 2011. Dry matter and nitrogen accumulation and partitioning in rice (Oryza sativa L.) exposed to experimental warming with elevated CO2. Plant Soil, 342(1/2): 59-71. |
| [15] | Li G, Kuijer H N J, Yang X J, Liu H R, Shen C Q, Shi J, Betts N, Tucker M R, Liang W Q, Waugh R, Burton R A, Zhang D B. 2021. MADS1 maintains barley spike morphology at high ambient temperatures. Nat Plants, 7(8): 1093-1107. |
| [16] | Ling C H, Su Z F, Chang H C, Cai J Z, Ho J S. 1983. The leaf-age model of development process in different cultivars of rice. Sci Agric Sin, 16: 9-18. (in Chinese with English abstract) |
| [17] | Liu Q Y, Li M, Ji X, Liu J, Wang F J, Wei Y F. 2022. Characteristics of grain yield, dry matter production and nitrogen uptake and transport of rice varieties with different grain protein content. Agronomy, 12(11): 2866. |
| [18] | Loladze I, Nolan J M, Ziska L H, Knobbe A R. 2019. Rising atmospheric CO2lowers concentrations of plant carotenoids essential to human health: A meta-analysis. Mol Nutr Food Res, 63(15): 1801047. |
| [19] | Madan P, Jagadish S K, Craufurd P Q, Fitzgerald M, Lafarge T, Wheeler T R. 2012. Effect of elevated CO2 and high temperature on seed-set and grain quality of rice. J Exp Bot, 63(10): 3843-3852. |
| [20] | Mae T, Ohira K. 1981. The remobilization of nitrogen related to leaf growth and senescence in rice plants (Oryza sativa L.). Plant Cell Physiol, 22(6): 1067-1074. |
| [21] | Ntanos D A, Koutroubas S D. 2002. Dry matter and N accumulation and translocation for Indica and Japonica rice under Mediterranean conditions. Field Crops Res, 74(1): 93-101. |
| [22] | Pan X Q, Welti R, Wang X M. 2010. Quantitative analysis of major plant hormones in crude plant extracts by high-performance liquid chromatography-mass spectrometry. Nat Protoc, 5(6): 986-992. |
| [23] | Peng S B, Buresh R J, Huang J L, Zhong X H, Zou Y B, Yang J C, Wang G H, Liu Y Y, Hu R F, Tang Q Y, Cui K H, Zhang F S, Dobermann A. 2010. Improving nitrogen fertilization in rice by sitespecific N management. A review. Agron Sustain Dev, 30(3): 649-656. |
| [24] | Radley M. 1981. The effect on wheat grain growth of the removal or ABA treatment of glumes and lemmas. J Exp Bot, 32(1): 129-140. |
| [25] | Schaarschmidt S, Lawas L M F, Kopka J, Jagadish S V K, Zuther E. 2021. Physiological and molecular attributes contribute to high night temperature tolerance in cereals. Plant Cell Environ, 44(7): 2034-2048. |
| [26] | Shi P H, Zhu Y, Tang L, Chen J L, Sun T, Cao W X, Tian Y C. 2016. Differential effects of temperature and duration of heat stress during anthesis and grain filling stages in rice. Environ Exp Bot, 132: 28-41. |
| [27] | Shi W J, Muthurajan R, Rahman H, Selvam J, Peng S B, Zou Y B, Jagadish K S V. 2013. Source-sink dynamics and proteomic reprogramming under elevated night temperature and their impact on rice yield and grain quality. New Phytol, 197(3): 825-837. |
| [28] | Shi W J, Yin X Y, Struik P C, Xie F M, Schmidt R C, Jagadish K S V. 2016. Grain yield and quality responses of tropical hybrid rice to high night-time temperature. Field Crops Res, 190: 18-25. |
| [29] | Shi W J, Yin X Y, Struik P C, Solis C, Xie F M, Schmidt R C, Huang M, Zou Y B, Ye C R, Jagadish S V K. 2017. High day- and night-time temperatures affect grain growth dynamics in contrasting rice genotypes. J Exp Bot, 68(18): 5233-5245. |
| [30] | Sreenivasulu N, Butardo V M Jr, Misra G, Cuevas R P, Anacleto R, Kavi Kishor P B. 2015. Designing climate-resilient rice with ideal grain quality suited for high-temperature stress. J Exp Bot, 66(7): 1737-1748. |
| [31] | Sun P Y, Zhang W H, Wang Y H, He Q, Shu F, Liu H, Wang J, Wang J M, Yuan L P, Deng H F. 2016. OsGRF4 controls grain shape, panicle length and seed shattering in rice. J Integr Plant Biol, 58(10): 836-847. |
| [32] | Sun T, Hasegawa T, Tang L, Wang W, Zhou J J, Liu L L, Liu B, Cao W X, Zhu Y. 2018. Stage-dependent temperature sensitivity function predicts seed-setting rates under short-term extreme heat stress in rice. Agric For Meteorol, 256/257: 196-206. |
| [33] | Sun T, Liu B, Hasegawa T, Liao Z Y, Tang L, Liu L L, Cao W X, Zhu Y. 2023. Sink-source unbalance leads to abnormal partitioning of biomass and nitrogen in rice under extreme heat stress: An experimental and modeling study. Eur J Agron, 142: 126678. |
| [34] | Tabuchi H, Zhang Y, Hattori S, Omae M, Shimizu-Sato S, Oikawa T, Qian Q, Nishimura M, Kitano H, Xie H, Fang X H, Yoshida H, Kyozuka J, Chen F, Sato Y. 2011. LAX PANICLE2 of rice encodes a novel nuclear protein and regulates the formation of axillary meristems. Plant Cell, 23(9): 3276-3287. |
| [35] | Tang S, Zhang H X, Liu W Z, Dou Z, Zhou Q Y, Chen W Z, Wang S H, Ding Y F. 2019. Nitrogen fertilizer at heading stage effectively compensates for the deterioration of rice quality by affecting the starch-related properties under elevated temperatures. Food Chem, 277: 455-462. |
| [36] | Tu D B, Jiang Y, Salah A, Cai M L, Peng W, Zhang L J, Li C F, Cao C G. 2022. Response of source-sink characteristics and rice quality to high natural field temperature during reproductive stage in irrigated rice system. Front Plant Sci, 13: 911181. |
| [37] | Wang W L, Cai C, Lam S K, Liu G, Zhu J G. 2018. Elevated CO2 cannot compensate for japonica grain yield losses under increasing air temperature because of the decrease in spikelet density. Eur J Agron, 99: 21-29. |
| [38] | Wang W L, Xu X, Zhu C W, Gu J F, Zhang W Y, Liu G, Zhu J G. 2019. Elevated CO2-induced changes in cytokinin and nitrogen metabolism are associated with different responses in the panicle architecture of two contrasting rice genotypes. Plant Growth Regul, 89(2): 119-129. |
| [39] | Wang W L, Cai C, He J, Gu J F, Zhu G L, Zhang W Y, Zhang J G, Liu G. 2020. Yield, dry matter distribution and photosynthetic characteristics of rice under elevated CO2 and increased temperature conditions. Field Crops Res, 248: 107605. |
| [40] | Wang W L, Huang L Y, Zhu G L, Zhang H, Wang Z Q, Adnan M, Saud S, Hayat Z, Fahad S. 2022. Screening of rice cultivars for nitrogen use efficiency and yield stability under varying nitrogen levels. J Plant Growth Regul, 41(4): 1808-1819. |
| [41] | Wang Y L, Zhang Y K, Shi Q H, Chen H Z, Xiang J, Hu G H, Chen Y H, Wang X D, Wang J K, Yi Z H, Zhu D F, Zhang Y P. 2020. Decrement of sugar consumption in rice young panicle under high temperature aggravates spikelet number reduction. Rice Sci, 27(1): 44-55. |
| [42] | Wang Z Q, Zhang W Y, Beebout S S, Zhang H, Liu L J, Yang J C, Zhang J H. 2016. Grain yield, water and nitrogen use efficiencies of rice as influenced by irrigation regimes and their interaction with nitrogen rates. Field Crops Res, 193: 54-69. |
| [43] | Wang Z Q, Zhang W Y, Yang J C. 2018. Physiological mechanism underlying spikelet degeneration in rice. J Integr Agric, 17(7): 1475-1481. |
| [44] | Wei L L, Wang W L, Zhu J G, Wang Z Q, Wang J Q, Li C H, Zeng Q, Ziska L H. 2021. Responses of rice qualitative characteristics to elevated carbon dioxide and higher temperature: Implications for global nutrition. J Sci Food Agric, 101(9): 3854-3861. |
| [45] | Wu C, Cui K H, Wang W C, Li Q, Fahad S, Hu Q Q, Huang J L, Nie L X, Mohapatra P K, Peng S B. 2017. Heat-induced cytokinin transportation and degradation are associated with reduced panicle cytokinin expression and fewer spikelets per panicle in rice. Front Plant Sci, 8: 371. |
| [46] | Wu C, Tang S, Li G H, Wang S H, Fahad S, Ding Y F. 2019. Roles of phytohormone changes in the grain yield of rice plants exposed to heat: A review. PeerJ, 7: e7792. |
| [47] | Wu C, Cui K H, Li Q, Li L Y, Wang W C, Hu Q Q, Ding Y F, Li G H, Fahad S, Huang J L, Nie L X, Peng S B. 2021. Estimating the yield stability of heat-tolerant rice genotypes under various heat conditions across reproductive stages: A 5-year case study. Sci Rep, 11: 13604. |
| [48] | Wu C, Song Y J, Qi B B, Fahad S. 2023. Effects of asymmetric heat on grain quality during the panicle initiation stage in contrasting rice genotypes. J Plant Growth Regul, 42(2): 630-636. |
| [49] | Xiong D L, Ling X X, Huang J L, Peng S B. 2017. Meta-analysis and dose-response analysis of high temperature effects on rice yield and quality. Environ Exp Bot, 141: 1-9. |
| [50] | Yang J C, Zhang J H. 2010. Crop management techniques to enhance harvest index in rice. J Exp Bot, 61(12): 3177-3189. |
| [51] | Yang L X, Huang J Y, Yang H J, Dong G H, Liu G, Zhu J G, Wang Y L. 2006. Seasonal changes in the effects of free-air CO2 enrichment (FACE) on dry matter production and distribution of rice (Oryza sativa L.). Field Crops Res, 98(1): 12-19. |
| [52] | Zhang H, Li H W, Yuan L M, Wang Z Q, Yang J C, Zhang J H. 2012. Post-anthesis alternate wetting and moderate soil drying enhances activities of key enzymes in sucrose-to-starch conversion in inferior spikelets of rice. J Exp Bot, 63(1): 215-227. |
| [53] | Zhang J, Li G H, Huang Q Y, Liu Z H, Ding C Q, Tang S, Chen L, Wang S H, Ding Y F, Zhang W J. 2017. Effects of culm carbohydrate partitioning on basal stem strength in a high-yielding rice population. Crop J, 5(6): 478-487. |
| [54] | Zhang W Y, Zhou Y J, Li C Q, Zhu K Y, Xu Y J, Wang W L, Liu L J, Zhang H, Gu J F, Wang Z Q, Zhang J H, Yang J C. 2022. Post- anthesis moderate soil-drying facilitates source-to-sink remobilization of nitrogen via redistributing cytokinins in rice. Field Crops Res, 288: 108692. |
| [55] | Zhao Y D. 2010. Auxin biosynthesis and its role in plant development. Annu Rev Plant Biol, 61: 49-64. |
| [56] | Zhen F X, Wang W, Wang H Y, Zhou J J, Liu B, Zhu Y, Liu L L, Cao W X, Tang L. 2019. Effects of short-term heat stress at booting stage on rice-grain quality. Crop Pasture Sci, 70(6): 486-498. |
| [57] | Zhen F X, Liu Y J, Ali I, Liu B, Liu L L, Cao W X, Tang L, Zhu Y. 2020a. Short-term heat stress at booting stage inhibited nitrogen remobilization to grain in rice. J Agric Food Res, 2: 100066. |
| [58] | Zhen F X, Zhou J J, Mahmood A, Wang W, Chang X N, Liu B, Liu L L, Cao W X, Zhu Y, Tang L. 2020b. Quantifying the effects of short-term heat stress at booting stage on nonstructural carbohydrates remobilization in rice. Crop J, 8(2): 194-212. |
| [59] | Zhong L J, Cheng F M, Wen X, Sun Z X, Zhang G P. 2005. The deterioration of eating and cooking quality caused by high temperature during grain filling in early-season indica rice cultivars. J Agron Crop Sci, 191(3): 218-225. |
| [60] | Zhu K Y, Zhou Q, Shen Y, Yan J Q, Xu Y J, Wang Z Q, Yang J C. 2020. Agronomic and physiological performance of an indica- japonica rice variety with a high yield and high nitrogen use efficiency. Crop Sci, 60(3): 1556-1568. |
| [61] | Zhu K Y, Yan J Q, Y, Zhang W Y, Xu Y J, Wang Z Q, Yang J C. 2022. Deciphering the morpho-physiological traits for high yield potential in nitrogen efficient varieties (NEVs): A japonica rice case study. J Integr Agric, 21(4): 947-963. |
| [62] | Zhu L J, Gu M H, Meng X L, Cheung S C K, Yu H X, Huang J, Sun Y, Shi Y C, Liu Q Q. 2012. High-amylose rice improves indices of animal health in normal and diabetic rats. Plant Biotechnol J, 10(3): 353-362. |
| [1] | Prathap V, Suresh Kumar, Nand Lal Meena, Chirag Maheshwari, Monika Dalal, Aruna Tyagi. Phosphorus Starvation Tolerance in Rice Through Combined Physiological, Biochemical, and Proteome Analyses [J]. Rice Science, 2023, 30(6): 613-631. |
| [2] | Serena Reggi, Elisabetta Onelli, Alessandra Moscatelli, Nadia Stroppa, Matteo Dell’Anno, Kiril Perfanov, Luciana Rossi. Seed-Specific Expression of Apolipoprotein A-IMilano Dimer in Engineered Rice Lines [J]. Rice Science, 2023, 30(6): 587-597. |
| [3] | Sundus Zafar, Xu Jianlong. Recent Advances to Enhance Nutritional Quality of Rice [J]. Rice Science, 2023, 30(6): 523-536. |
| [4] | Kankunlanach Khampuang, Nanthana Chaiwong, Atilla Yazici, Baris Demirer, Ismail Cakmak, Chanakan Prom-U-Thai. Effect of Sulfur Fertilization on Productivity and Grain Zinc Yield of Rice Grown under Low and Adequate Soil Zinc Applications [J]. Rice Science, 2023, 30(6): 632-640. |
| [5] | Fan Fengfeng, Cai Meng, Luo Xiong, Liu Manman, Yuan Huanran, Cheng Mingxing, Ayaz Ahmad, Li Nengwu, Li Shaoqing. Novel QTLs from Wild Rice Oryza longistaminata Confer Strong Tolerance to High Temperature at Seedling Stage [J]. Rice Science, 2023, 30(6): 577-586. |
| [6] | Lin Shaodan, Yao Yue, Li Jiayi, Li Xiaobin, Ma Jie, Weng Haiyong, Cheng Zuxin, Ye Dapeng. Application of UAV-Based Imaging and Deep Learning in Assessment of Rice Blast Resistance [J]. Rice Science, 2023, 30(6): 652-660. |
| [7] | Md. Forshed Dewan, Md. Ahiduzzaman, Md. Nahidul Islam, Habibul Bari Shozib. Potential Benefits of Bioactive Compounds of Traditional Rice Grown in South and Southeast Asia: A Review [J]. Rice Science, 2023, 30(6): 537-551. |
| [8] | Raja Chakraborty, Pratap Kalita, Saikat Sen. Phenolic Profile, Antioxidant, Antihyperlipidemic and Cardiac Risk Preventive Effect of Pigmented Black Rice Variety Chakhao poireiton in High-Fat High-Sugar Induced Rats [J]. Rice Science, 2023, 30(6): 641-651. |
| [9] | Li Qianlong, Feng Qi, Wang Heqin, Kang Yunhai, Zhang Conghe, Du Ming, Zhang Yunhu, Wang Hui, Chen Jinjie, Han Bin, Fang Yu, Wang Ahong. Genome-Wide Dissection of Quan 9311A Breeding Process and Application Advantages [J]. Rice Science, 2023, 30(6): 552-565. |
| [10] | Nazaratul Ashifa Abdullah Salim, Norlida Mat Daud, Julieta Griboff, Abdul Rahim Harun. Elemental Assessments in Paddy Soil for Geographical Traceability of Rice from Peninsular Malaysia [J]. Rice Science, 2023, 30(5): 486-498. |
| [11] | Ammara Latif, Sun Ying, Pu Cuixia, Noman Ali. Rice Curled Its Leaves Either Adaxially or Abaxially to Combat Drought Stress [J]. Rice Science, 2023, 30(5): 405-416. |
| [12] | Liu Qiao, Qiu Linlin, Hua Yangguang, Li Jing, Pang Bo, Zhai Yufeng, Wang Dekai. LHD3 Encoding a J-Domain Protein Controls Heading Date in Rice [J]. Rice Science, 2023, 30(5): 437-448. |
| [13] | Lu Xuedan, Li Fan, Xiao Yunhua, Wang Feng, Zhang Guilian, Deng Huabing, Tang Wenbang. Grain Shape Genes: Shaping the Future of Rice Breeding [J]. Rice Science, 2023, 30(5): 379-404. |
| [14] | Zhang Guomei, Li Han, Liu Shanshan, Zhou Xuming, Lu Mingyang, Tang Liang, Sun Lihua. Water Extract of Rice False Smut Balls Activates Nrf2/HO-1 and Apoptosis Pathways, Causing Liver Injury [J]. Rice Science, 2023, 30(5): 473-485. |
| [15] | Tan Jingyi, Zhang Xiaobo, Shang Huihui, Li Panpan, Wang Zhonghao, Liao Xinwei, Xu Xia, Yang Shihua, Gong Junyi, Wu Jianli. ORYZA SATIVA SPOTTED-LEAF 41 (OsSPL41) Negatively Regulates Plant Immunity in Rice [J]. Rice Science, 2023, 30(5): 426-436. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||